Expertise Library
How water activity and pH work together to control microbial growth

Using both pH and aW controls microbes more effectively than just one or the other. Here's how food manufacturers can use hurdle technology to improve formulations.
Water activity and pH are the two most important intrinsic factors that determine if a product will support the growth of a spoilage microorganism. Water activity and pH work synergistically, with their combined effects being more powerful at control than their individual effects. This synergistic effect is described in detail by hurdle technology for microbial control and is an intricate part of the FDA’s definition of potentially hazardous foods.
Here’s how you can use the combined power of water activity and pH to increase microbial control using milder preservation techniques, which may result in improved product texture and quality.
How water activity prevents microbial growth
Like all organisms, microorganisms rely on water for growth. They take up water by moving it across the cell membrane. This water movement mechanism depends on a water activity gradient—on water moving from a high water activity environment outside the cell to a lower water activity environment within the cell.
When water activity outside the cell becomes low enough, it causes osmotic stress: the cell cannot take up water and becomes dormant. The microorganisms are not eliminated, they just become unable to reproduce. Different organisms cope with osmotic stress in different ways. That’s why there are different growth limits for each organism. Some types of molds and yeasts have adapted to withstand very low water activity levels.
Each organism has a specific water activity at which it will stop growing. As long as product developers keep the water activity below this limit, the microbe in question won’t replicate to high enough levels to cause infection or illness. See Table 1.
Microbial growth limits make water activity an excellent tool for assuring the safety of food products, and water activity measurement can be used as a critical control point in HACCP plans.
Opportunities for synergy
The growth limits in Table 1 assume that all other conditions (pH, temperature, etc.) are optimal for the growth of the organism. If the growth-limiting effects of lowered pH are combined with water activity control, however, microbial growth can actually be controlled at a higher water activity than shown on the chart.
What is pH?
pH is a measure of the degree of acidity or alkalinity of a solution. Values between 0 and 7 indicate acidity; values between 7 and 14 indicate alkalinity. Distilled water, which is neutral, has a pH of 7. Foods tend to be either neutral or acidic.
Microbes have pH growth limits
Just as with water activity, microorganisms have pH limits below which they will not grow. Table 2 shows the minimum pH limits for the growth of different types of microorganisms. All microorganisms prefer a neutral pH for optimum growth, but they can grow in more acidic pH values. Most of them stop growing at a pH of 5.0. Some microorganisms can go as low as 4.6 and even down to 4.4. Historically, a pH of 4.6 was considered to be the lower growth limit, but portions of the food code were changed when it was discovered that some problematic microbes can grow in pH levels as low as 4.2.
Uses for pH adjustment
Because of microbial growth limits, lowering pH is an effective way to preserve foods and prevent the growth of microorganisms and can also be used as a critical control point in HAACP plans. Additionally, some manufacturers adjust pH to change flavor. This is often done through pickling or fermentation, which use microbial action, enzymatic reactions, or acids such as vinegar to induce the production of lactic acid. Many chemical reactions are pH dependent and can be prevented or controlled by adjusting pH.
Water activity and pH—more powerful together
The effects of water activity and pH can be combined through hurdle technology to control microorganisms more effectively. In the case of water activity and pH, the combined effect of both hurdles is greater than the effects each hurdle alone. This means you can have effective microbial control at levels that would typically be considered unsafe for either pH or for water activity alone. The currently valid 2013 food code contains pH and water activity interaction tables, shown in Tables 3 and 4, that can be used to determine whether or not a food requires time and temperature control for safety (TCS).
Table 3 applies to foods that have been heat-treated to destroy microorganisms and then packaged. Lowered water activity and pH are not kill steps. They do not eliminate microorganisms. They simply prevent growth of microbes to toxic levels. Because the heat treatment destroys all microorganisms except spore forming bacteria, they can be packaged at higher water activities and pH levels. Under these conditions, a water activity of 0.92 and a pH of 4.6 or greater is considered safe.
Interactive Table 4 is used for products that are not heat treated, or that are heat-treated and unpackaged. Typically, these products require a water activity of less than 0.88 or a pH level of less than 4.2 to be considered safe. However, higher values can be acceptable when water activity and pH are combined.
Table 5 shows the water activity and pH of some common foods. Strawberry preserves have a very high water activity, but the pH is quite low. Because of the citric acid present, the pH is low enough to prevent microbial growth even though the water activity is high. Mustard also has a very low pH but a high water activity. These products are safe because of their pH, not because of their water activity. Maple syrup is high in sugar, so it’s lower in water activity, but its pH is fairly neutral. In this case, it’s the water activity that would provide the safety, not the pH.
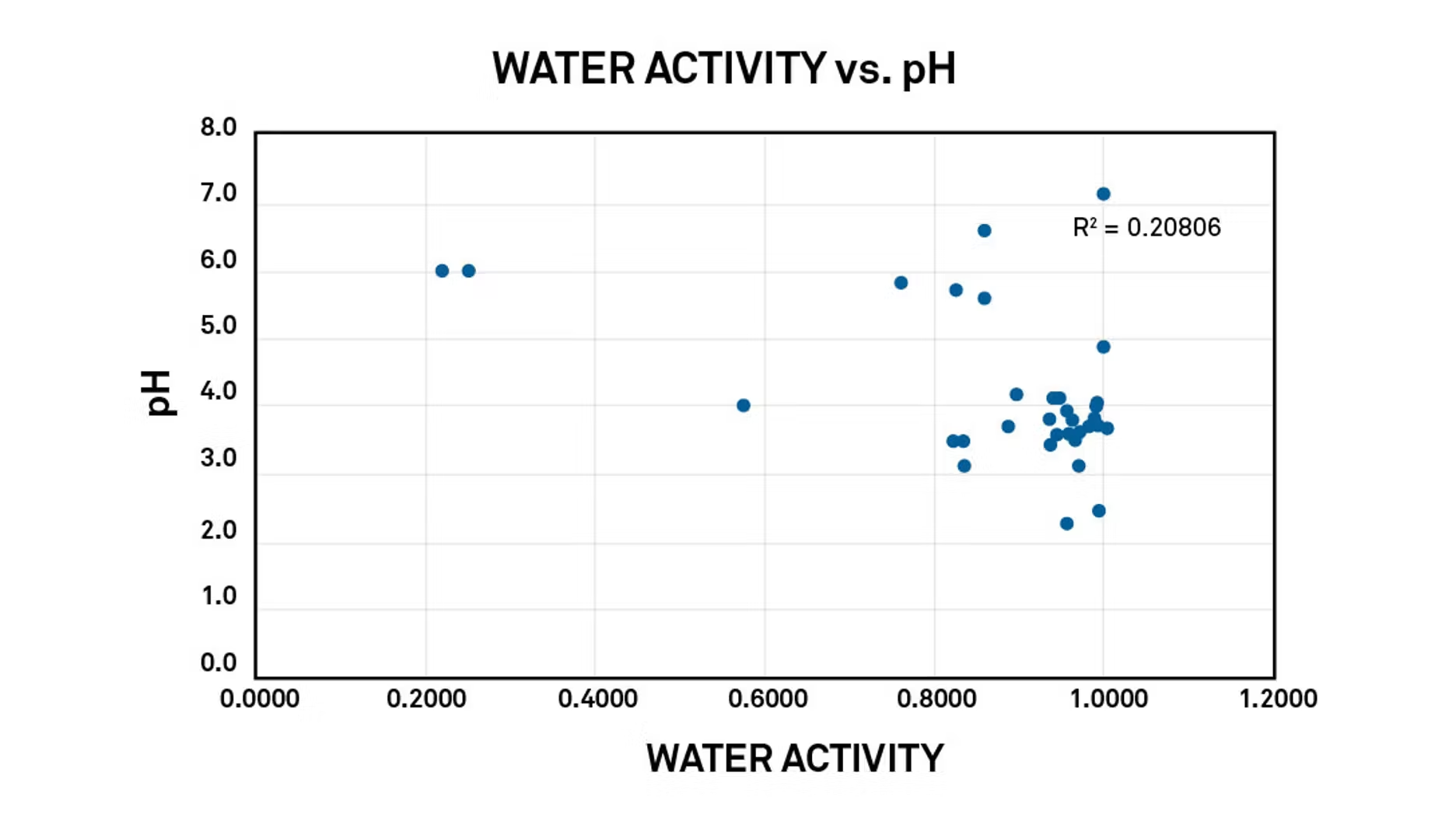
Figure 1 shows that if water activity and pH are plotted together, there isn’t any kind of direct relationship. If acid is added to a product to lower its pH, it will have some impact on water activity because acidic materials tend to be polar, and they preferentially interact with water. But essentially, lowering the pH will not directly lower the water activity.
How to control water activity
The most common way to lower a product’s water activity is to dry or bake it. (Although note that to do this properly, you must first understand the product’s moisture sorption isotherm). Water activity can also be controlled by adding humectants like salt, sugar, high fructose corn syrup, sorbitol, and maltodextrin.
Common ways to control pH
The most common way to lower pH is through fermentation. Fermentation relies on “good” bacteria to produce lactic acid, which then lowers the pH of the product and prevents the growth of other types of organisms. Pickles, sauerkraut, fermented sausages, and olives all use this strategy. pH can also be controlled by adding acid (vinegar, lactic acid, citric acid) directly to the product, or by adding naturally acidic ingredients like tomatoes in spaghetti sauce.
Water activity and pH—fast and easy measurements
Water activity and pH are two measurements that are better together. And both are easy to measure using readily available commercial water activity meters and pH meters.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
