Education Guides
The food manufacturer’s complete guide to water activity (aw)

For a cheap ingredient, water can cause a lot of expensive problems in the food industry. The best way to understand water in your product is by learning about water activity (aw).
Why water activity?
Water activity’s usefulness as a quality and safety measurement was suggested when it became evident moisture content could not adequately account for microbial growth fluctuations. The water activity (aw) concept has served the microbiologist and food technologist for decades. It is the most commonly used criterion for safety and quality.
Water activity: it’s all about energy
What is water activity?
Take a glass of water and a dry sponge. Dip the corner of the sponge into the glass of water. The water will move from the glass into the sponge.
Water activity is the force that causes the water to move into the sponge. To understand it better, think about how the water in the sponge is different from the water in the glass.
The water in the glass is free, but the water in the sponge is anything but free. It’s bound by hydrogen bonds, capillary forces, and van der Waals-London forces. These are called matrix effects. The water in the sponge has a lower energy state than the water in the glass. Water will flow into the sponge, but to get it back out, we must do work by squeezing the sponge.
The water in the sponge has a lower vapor pressure, lower freezing point, and higher boiling point than the water in the glass. They are different in ways we can measure and quantify.
Water’s energy can also be decreased by diluting it with solutes. These are called osmotic effects. Since work is required to restore the water to its pure, free state, this also reduces the water activity. The total change in energy is the sum of matric and osmotic effects.
Water activity controls food quality and safety
Combine crackers at 20% water content and cheese filling at 30%. A recipe for soggy crackers? Not if the two ingredients have the same water activity. Need to avoid clumping and caking in a batch of spices? Match the water activities of the components, and the problem is solved.
Vitamin degradation is a function of water activity. So are lipid oxidation, crunchiness, chewiness, softness, and many other factors of quality. Moisture content will tell you how much water is in a product, but that’s all. It can’t predict any of these other quality and safety issues.
Predicting safety and stability
Water activity predicts safety and stability with respect to microbial growth, chemical and biochemical reaction rates, and physical properties. Figure 1 shows stability in terms of microbial growth limits and rates of degradative reactions as a function of water activity.
By measuring and controlling the water activity, it is possible to:
- Predict which microorganisms will be potential sources of spoilage and infection
- Maintain the chemical stability of products
- Minimize nonenzymatic browning reactions and spontaneous autocatalytic lipid oxidization reactions
- Prolong the activity of enzymes and vitamins
- Optimize the physical properties of products for moisture migration, texture, and shelf life
How is water activity measured?
If we enclose a sample in a sealed container, the relative humidity of the air in the headspace will equilibrate with the water activity of the sample. At equilibrium, the two will be equal, and we can measure the relative humidity of the headspace to know the water activity of the sample. This is the most reliable answer to the question of how to measure water activity.
Secondary methods: hygrometers, capacitance sensors
Like early water activity meters, most modern instruments use electrical capacitance or resistance hygrometer sensors to measure humidity in the headspace above the sample.
These meters use secondary methods: they relate an electrical signal to relative humidity and must be calibrated with known salt standards.
With these sensors, the ERH is equal to the sample water activity only as long as the sample and sensor temperatures are the same. Accurate measurements require good temperature control or measurement. Capacitance sensors use a simple design and are often used in relatively inexpensive water activity meters.
Dew point is a primary method
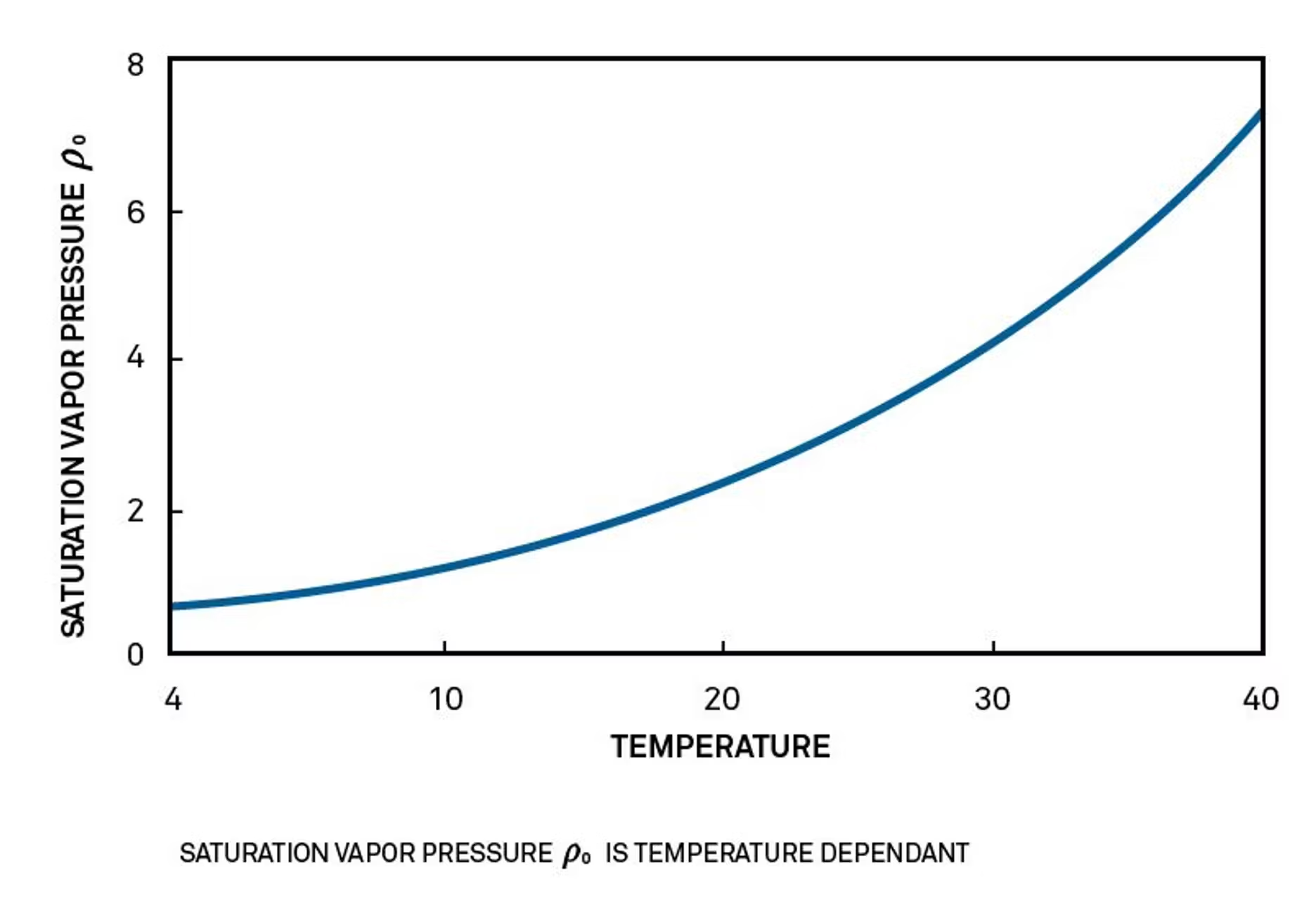
The best methods that answer the question of how to measure water activity are primary methods that use the ratio p /p0.P0 (the saturation vapor pressure) depends only on the temperature of the sample (as shown in the accompanying graph), so it’s possible to measure p0 by measuring the temperature of the sample. P (the vapor pressure of the water in the sample) can be measured by measuring the vapor pressure of water in the sealed head space above the sample. The most accurate way of measuring that vapor pressure, and one that goes back to first principles, is to measure the dew point of the air.
Primary method means direct measurement, no calibration
The major advantages of the dew point (or chilled mirror dew point) method are speed and accuracy. The chilled mirror dew point sensor is a primary measurement method based on fundamental thermodynamic principles. Chilled mirror water activity meters make highly accurate (±0.003aw) measurements, typically in about 5 minutes. Since the measurement is based on temperature determination, no calibration is necessary. Users should measure a standard salt solution to verify the proper functioning of the instrument. For some applications, the speed of this method allows manufacturers to perform at-line monitoring of a product’s water activity.
The AQUALAB 4TE uses the chilled-mirror dew point method which is shown in the literature to be the fastest most accurate water activity method on the market. Watch the video to see how it works.
Government regulations recommend the use of water activity
The U.S. Food and Drug Administration has incorporated water activity into safety regulations. These regulations detail specific requirements and practices to ensure that products are produced under sanitary conditions and are pure, wholesome, and safe. Use the table below as a reference to U.S. government safety regulations that recommend the use of water activity.
Water Activity Simplifies Shelf Life
Without an accurate, product-specific shelf life, you could be scrapping expired product that is still good. Or selling unexpired product that is actually bad. You could be paying too much for packaging that doesn’t help your product. Or giving up significant shelf life that would come from better packaging. The point is, you don’t know for sure because you’re operating in the dark.
So why don’t people do more shelf life testing?
Typically, it’s because a true, full-blown shelf life testing is a daunting task. It involves complex relationships between moisture, temperature, and product failure modes.
Any number of things could make your product unsafe or unpalatable—mold, microbial growth, rancidity, changes in texture or flavor, vitamin degradation. Most people don’t have the expertise to do full-blown shelf life testing in-house, and getting an outside lab to do it is expensive.
There is a scientifically sound alternative to this kind of shelf life testing. It’s shelf life, simplified by water activity. It generates all the data you need to predict your product’s shelf life from an experiment anyone, even a small startup, can afford to run.
Shelf life and water activity
How does water activity simplify shelf life?
- It eliminates distractions. When you know your product’s water activity, you will know which failure modes are an issue for that product.
- It simplifies prediction. You can use your water activity meter plus one other measurement method (which one depends on your particular failure mode) to run a straightforward, in-house experiment that will predict your shelf life accurately.
- It standardizes production. You can set a water activity specification that lets you achieve your optimal shelf life with every batch.
Your shelf life data can provide valuable insights to help you stop product failure, predict and lengthen shelf life, choose the most cost-effective packaging, and more.
Factors that end shelf life
There are three main factors that influence shelf life: microbial properties, chemical changes, and physical deterioration. All of these factors are connected to water activity.
Microbial growth
Microorganisms have a limiting water activity level below which they will not grow. Water activity, not moisture content, determines the lower limit of “available” water for microbial growth. Since bacteria, yeast, and molds require a certain amount of “available” water to support growth, designing a product below a critical water activity level provides an effective means to control growth.
Water may be present, even at high content levels, in a product, but if its energy level is sufficiently low, the microorganisms cannot remove the water to support their growth. This ‘desert-like’ condition creates an osmotic imbalance between the microorganisms and the local environment. Consequently, the microbes cannot grow.
Mold and microbial growth are the most dangerous threats to shelf life. Controlling water activity can inhibit or preclude microbial growth, extend shelf life, and allow some products to be safely stored without refrigeration. Using well-defined tables, you can set a water activity limit for your product and use this in shelf life testing.
Chemical degradation
Water activity influences deteriorative chemical reaction rates because water acts as a solvent, can be a reactant itself, or can change the mobility of reactants through viscosity. For example, non-enzymic browning reactions increase with increasing water activity to a maximum at 0.6 to 0.7 aw, and lipid oxidation is minimized from about 0.2 to 0.3 aw. Optimum chemical stability is generally found near the monolayer moisture content, as determined from moisture sorption isotherms.
Physical deterioration
High and (less often) low humidity environments can affect a product’s water activity, causing undesirable changes in the product’s texture or physical properties and shortening shelf life. Issues include loss of crispness in dry products, caking and clumping of powders, and toughness or chewiness in moist products. Finding the critical water activity for your product can involve some research, but water activity makes it much easier to do.

Packaging, shipping, and storage
Water activity changes during shipping and storage can profoundly influence shelf life. Water activity is a function of temperature, and shipping and storage temperatures can affect water activity inside the package. Simplified shelf life testing can help you determine the best packaging and evaluate the effect of shipping and storage conditions on the shelf life of your product.
Isotherms pinpoint your water activity sweet spot
Predict product changes over time
Food manufacturers need to know how long it will be before their product molds, gets soggy, goes stale, becomes rancid, cakes, clumps, crystallizes, and becomes unacceptable to the consumer. The moisture sorption isotherm is a powerful tool for predicting and extending the shelf life of a product. It allows you to:
- Find critical water activity values where changes like caking, clumping, and loss of texture occur
- Predict how the product will respond to ingredient and formulation changes
- Accurately estimate shelf life
- Create mixing models
- Perform packaging calculations
- Find the monolayer value (where a product is most stable)
Isotherms: the Holy Grail of formulation
A moisture sorption isotherm is a graph showing how water activity (aw) changes as water is adsorbed into and desorbed from a product held at constant temperature. This relationship is complex and unique for each product. Water activity almost always increases as moisture content increases, but the relationship is not linear. In fact, moisture sorption isotherms are S-shaped (sigmoidal) for most foods and J-shaped for foods that contain crystalline materials or high-fat content.
Handmade method impractical
The classic way to create an isotherm is to put the sample in a desiccator with a salt solution of known water activity until the sample’s weight stops changing. Then, the sample is weighed to determine water content. Each sample produces one point on the isotherm curve.
Because the process takes so long, curves were traditionally constructed using five or six data points with curve-fitting equations like GAB or BET.
A faster way to create isotherms
Creating moisture sorption isotherms by hand is painstaking. The method needed automation. The method first used—and still used by most vapor sorption instruments—is called DVS, or dynamic vapor sorption. A sample is exposed to a stream of humidity-controlled air while a microbalance measures tiny changes in weight as the product adsorbs or desorbs water. Once equilibrium is achieved, the instrument dynamically steps to the next preset humidity level. Tests take anywhere from two days to several weeks.
The DVS method works well for investigating the kinetics of sorption—what happens to a product as it is exposed to certain humidities and how fast it adsorbs or desorbs water. The DVS method is not very helpful in creating a high-resolution isotherm curve, however, as each equilibrium step produces just one point on the isotherm curve.
DDI isotherms reveal what hasn’t been seen before
The dynamic dew point isotherm (DDI) method was designed to solve this problem. It creates high-resolution isotherms that show detail in the adsorption and desorption curves by taking a snapshot of both water activity and moisture content (every 5 seconds) as the sample is exposed to humidified or desiccated air. DDI graphs contain hundreds of data points and show details not previously visible, such as critical points where caking, clumping, deliquescence, and loss of texture occur.
How isotherms are created
The AQUALAB VSA automatically delivers fast, high-resolution DDI and DVS isotherm graphs that change the way you understand your product. Dual testing modes and sophisticated modeling software turn your data into the solutions you need to manufacture, monitor, store, and ship a great product.
Turn isotherm data into solutions
The VSA comes with intuitive, full-featured modeling software. The Moisture Analysis Toolkit shows you how to turn your data into solutions using research-tested predictive models. You’ll find all the models you need in one place, organized in a simple-to-use program. Identify the critical humidity for glass transition, evaluate packaging performance, determine hygroscopicity, track hysteresis, predict coating breakdown, find susceptibility to caking/clumping, and more.
Isotherms identify critical water activity values
Despite double-bagging and issuing strict temperature storage guidelines, a spray-dried milk manufacturer still had problems with clumping.
When glass transition becomes a problem
When milk is spray-dried, rapid evaporation leaves the sugars in a glassy state. Glassy lactose has entirely different properties than crystalline lactose. Due to low mobility, particles don’t cake together or clump up while the powder is in a glassy state. The crystalline structure is a lower energy state, so there will always be some molecules in transition from glassy to crystalline. Problems occur when the rate of transition reaches a tipping point.
Water activity predicts transition rate
At 0.30 aw, it might take several years for the all the lactose to become crystalline. At 0.40 aw, it might take a month. Above 0.43, the transition will occur in a few hours. Once the lactose has crystallized, the powdered milk is permanently changed. It holds a dramatically different amount of water, it won’t dissolve, and it doesn’t taste right. In essence, it has been ruined.
DDI isotherms predict glass transition point
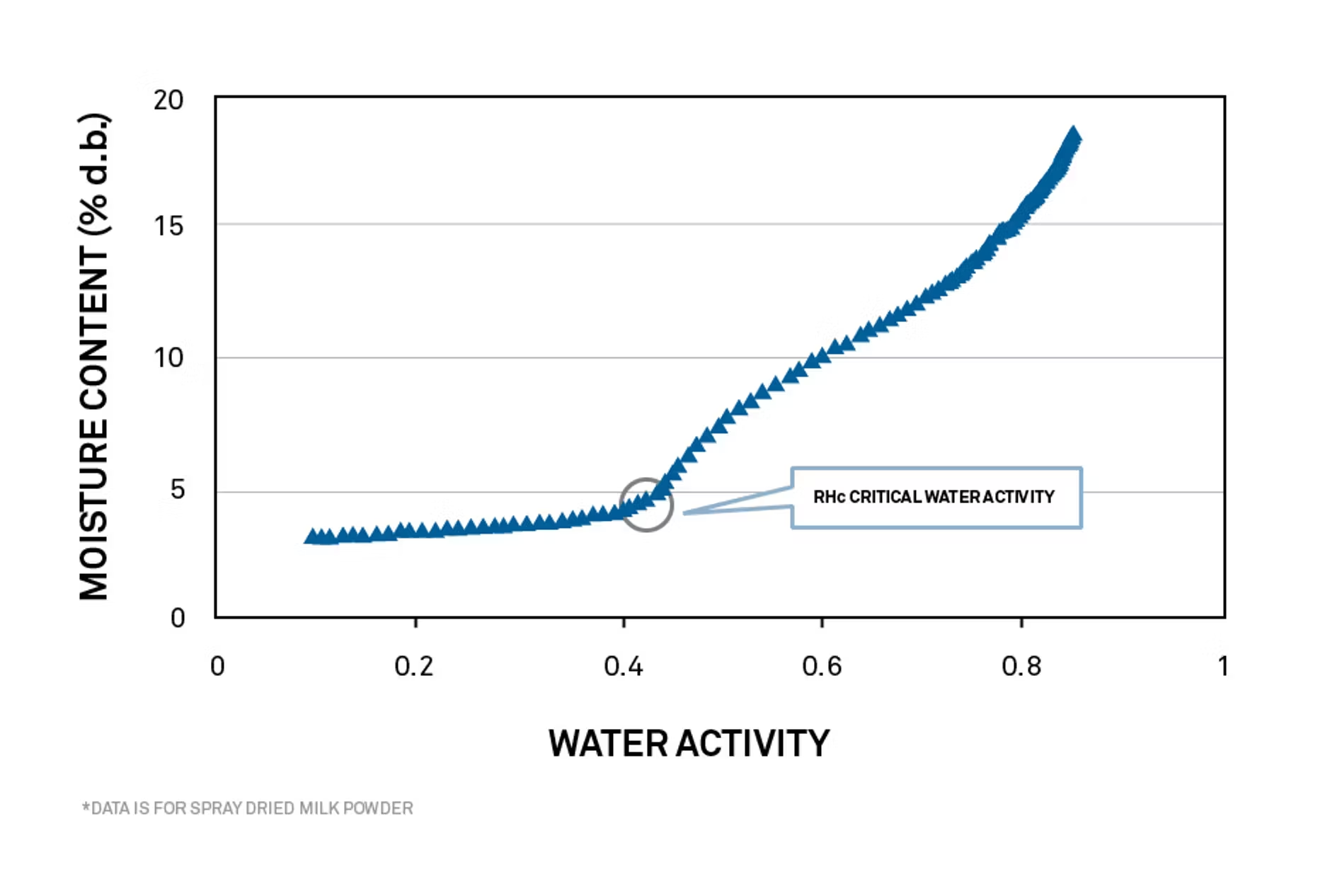
The glass transition point for powders like spray-dried milk can be determined using a high-resolution DDI isotherm. Traditional isotherms rely on models to fill in the isotherm between measured points. DDI isotherms measure hundreds of points and can identify transitions such as the glass transition point for spray-dried milk powder.
The peak value on the second derivative plot of the isotherm identifies the critical phase change value as 0.43 aw.
Routine, accurate testing at the line with better control values helped the manufacturer improve shipment acceptance rate.
Create mixing models
A cake manufacturer was formulating a recipe for cream-filled cake. The components of the recipe were frosting (about 7% moisture), cream filling (12%), and cake (15%). Moisture migration during shelf life had previously caused texture issues such as stale cake, rubbery frosting, and liquefied cream filling bleeding into the cake.
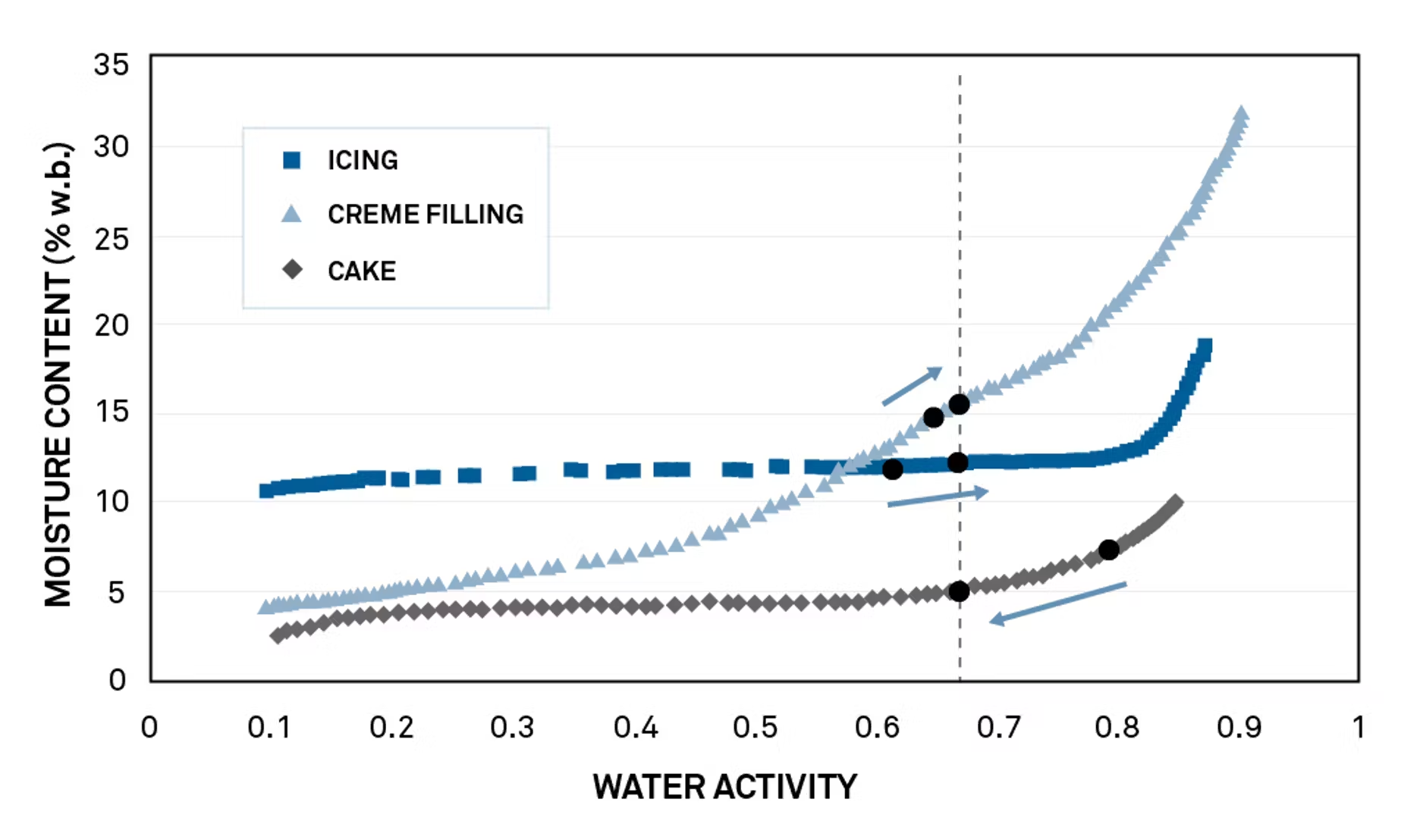
See how moisture will move between components
Moisture sorption isotherms for each ingredient showed that the frosting—the driest ingredient—had the highest water activity at 0.79. Water activities of the cream and the cake were similar—0.66 and 0.61 respectively.
Predict water activity of the final product
Transforming isotherms to chi plots predicted water activity of the final product as 0.67, a microbially safe value for the cake.
Avoid unpleasant surprises
The cake maker went on to successfully bake and taste test the cake at equilibrium water activity (0.67).
Select packaging
Single-serve powdered drink mixes are a growing market segment. Packaging accounts for more than 50% of the raw materials cost for this product. The main goal of the packaging is to maintain the drink mix below the critical aw over the target shelf life of the product.
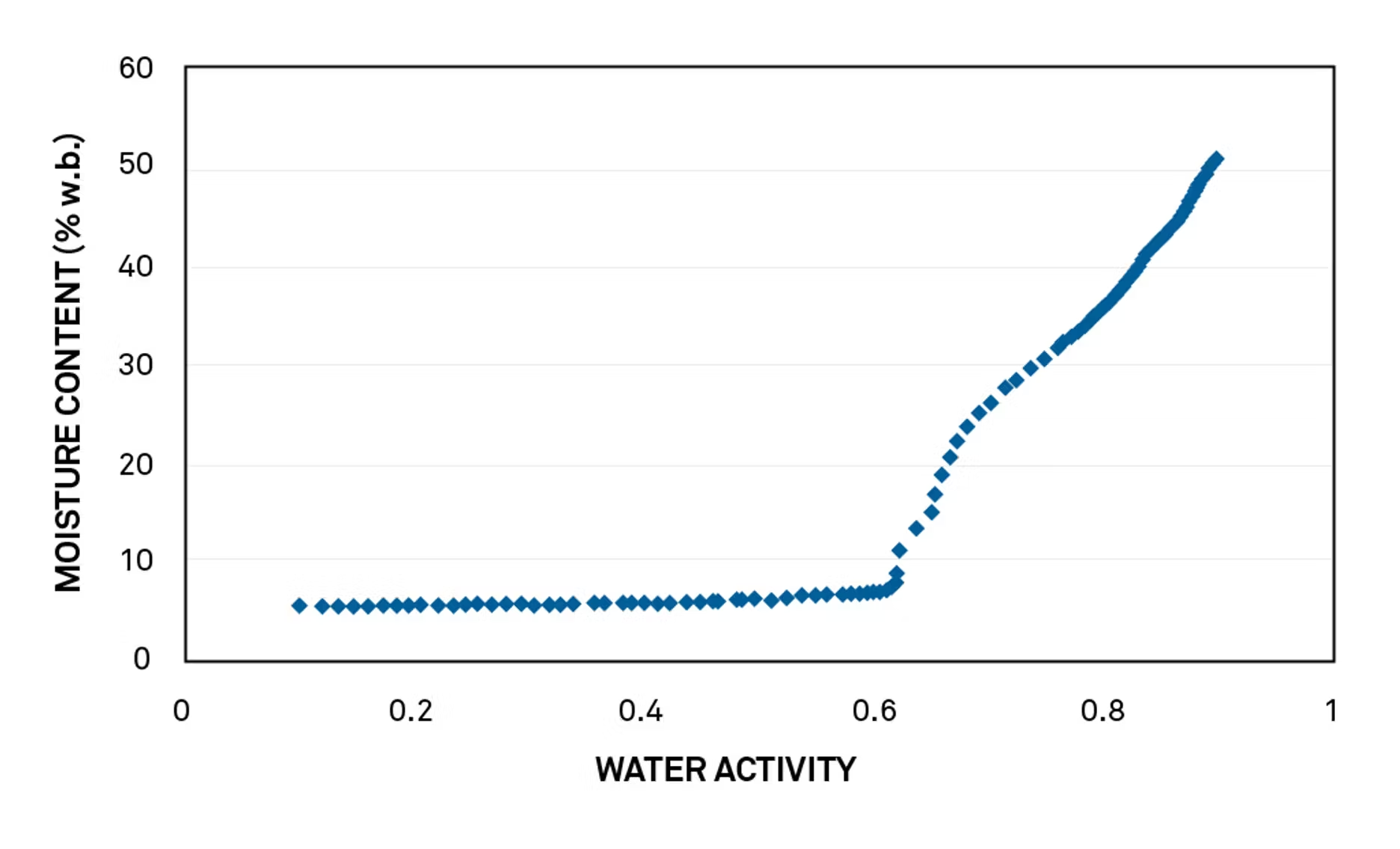
Packaging calculations begin with a critical water activity value. The ability to get a precise point from dynamic dew point isotherms (DDI) makes this type of packaging calculation possible.
This curve shows the glass transition point for a particular drink formulation:
The critical water activity—the exact inflection point—for this drink mix is 0.618 at 25° C.
Calculate package conductance
Using streamlined packaging calculations (available in Fundamentals of Isotherms and as a software tool), we evaluated four different types of packages for this drink mix—its original package and three possible alternatives. Under humidity abuse conditions (25° C, 75% humidity), here are the results:
Understand formulation changes
A pet food company changed formulation to produce a preservative-free product controlled by water activity. Shortly after introducing the product, they began to see returns due to spoilage.
Initial evaluation showed that spoilage predictions were based on water activity tests made at an unusually low temperature—15º C. Isotherms run at 15° C, 25° C, and 40° C showed that if stored under abuse conditions (as pet food often is), spoilage was likely.
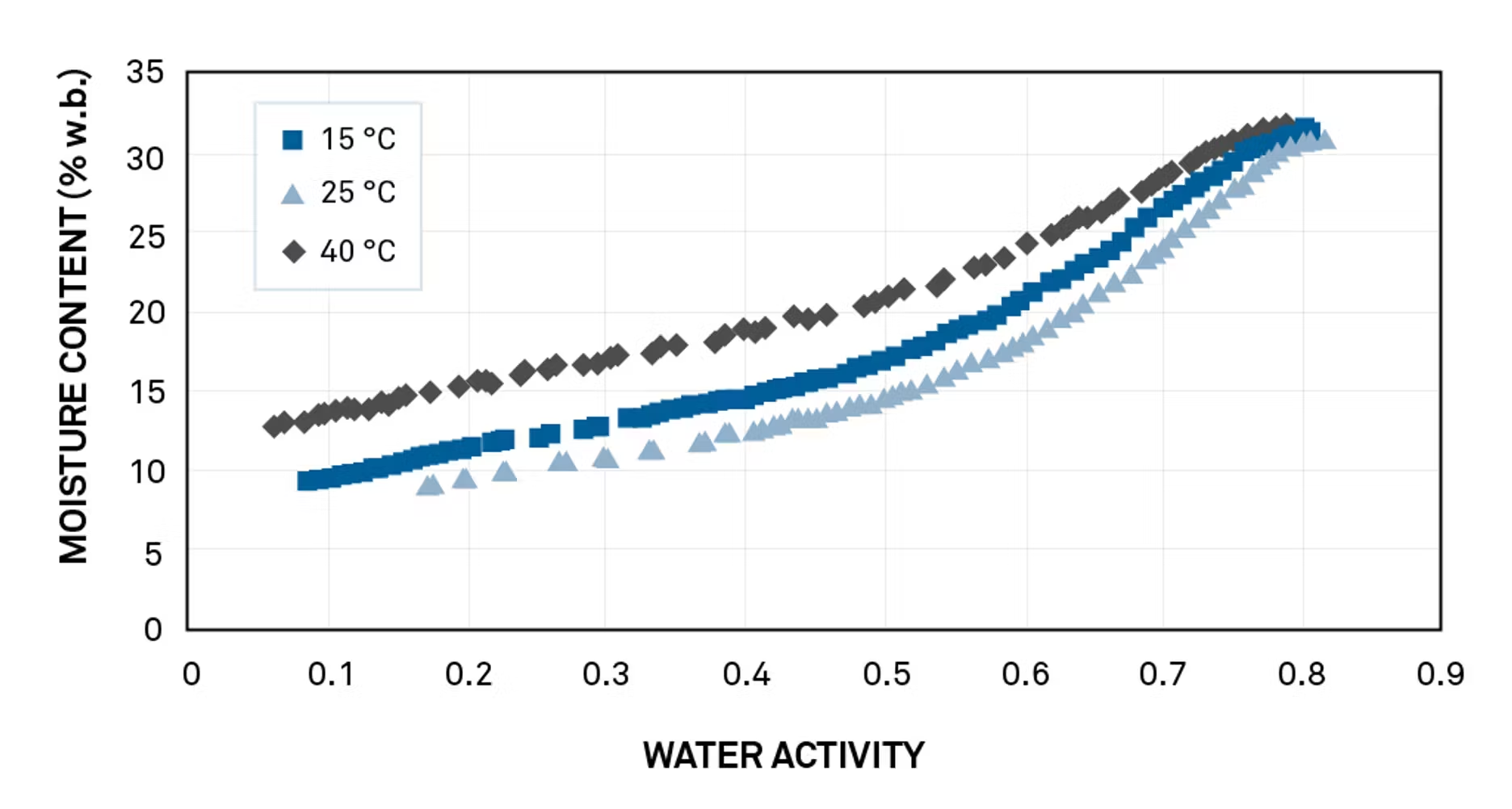
The isotherms offered a complete predictive picture, allowing the customer to solve the problem with a new formulation.
Investigate product failure
After 13 problem-free seasons, a pecan grower had his crop rejected because of mold issues. An isotherm was created to investigate the problem.
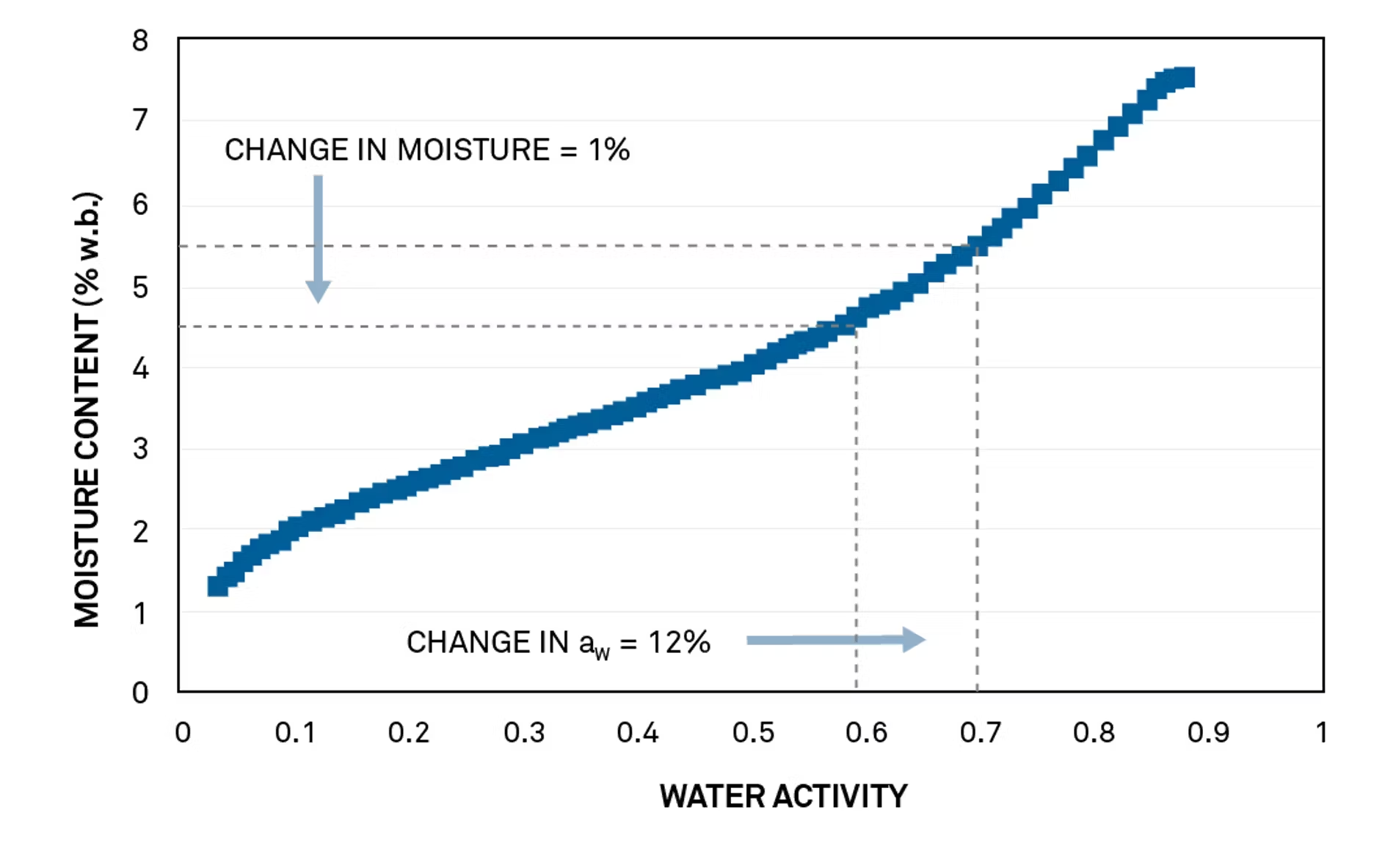
In order to avoid microbial growth, the pecans must be dried to 0.60 aw. As the isotherm shows, 0.60 aw corresponds to 4.8% mc in pecans. The pecan isotherm is also quite flat in this critical control region, so a small variation in moisture content translates into a large and potentially dangerous change in water activity.
Isotherm shows specs were set too low
The full isotherm shows that the pecan grower’s process was not adequate to guarantee the safety and quality of his crop. The pecan grower measured moisture content by loss on drying. Because his release specification was 5% and his accuracy was ± 0.5%, the dried crop’s actual water content could have been anywhere from 4.5% to 5.5%.
Anything from storage at high humidity to inadequate packaging could have pushed the pecans to unsafe water activities and resulted in spoilage.
Studies using dynamic dew point isotherms (DDI)
Allan, Matthew, and Lisa J. Mauer. “Comparison of methods for determining the deliquescence points of single crystalline ingredients and blends.” Food Chemistry 195 (2016): 29-38. doi:10.1016/j.foodchem.2015.05.042.
Allan, Matthew, Lynne S. Taylor, and Lisa J. Mauer. “Common-ion effects on the deliquescence lowering of crystalline ingredient blends.” Food Chemistry 195 (2016): 2-10. doi:10.1016/j.foodchem.2015.04.063.
Barry, Daniel M., and John W. Bassick. “NASA Space Shuttle Advanced Crew Escape Suit Development.” SAE Technical Paper Series, 1995. doi:10.4271/951545.
Bonner, Ian J., David N. Thompson, Farzaneh Teymouri, Timothy Campbell, Bryan Bals, and Jaya Shankar Tumuluru. “Impact of Sequential Ammonia Fiber Expansion (AFEX) Pretreatment and Pelletization on the Moisture Sorption Properties of Corn Stover.” Drying Technology 33, no. 14 (2015): 1768-778. doi:10.1080/07373937.2015.1039127.
Carter, B.P., Galloway, M.T., Campbell, G.S., and Carter, A.H. 2016. Changes in the moisture permeability of grain at the critical water activity from dynamic dew point isotherms. Transactions of the ASABE. 59(3):1023-1028.
Carter, B.P., Galloway, M.T., Morris, C.F., Weaver, G.L., and Carter, A.H. 2015. The case for water activity as a specification for wheat tempering and flour production. Cereal Foods World 60(4):166-170.
Carter, Brady P., Mary T. Galloway, Gaylon S. Campbell, and Arron H. Carter. “The critical water activity from dynamic dew point isotherms as an indicator of crispness in low moisture cookies.” Journal of Food Measurement and Characterization 9, no. 3 (2015): 463-70. doi:10.1007/s11694-015-9254-3.
Carter, Brady P., Mary T. Galloway, Gaylon S. Campbell, and Arron H. Carter. “The critical water activity from dynamic dew point isotherms as an indicator of pre-mix powder stability.” Journal of Food Measurement and Characterization 9, no. 4 (2015): 479-86. doi:10.1007/s11694-015-9256-1.
Carter, B.P and S.J. Schmidt. 2012. Developments in glass transition determination in foods using moisture sorption isotherms. Food Chemistry 132:1693-1698.
Coronel-Aguilera, Claudia P., and M. Fernanda San Martín-González. “Encapsulation of spray dried β-carotene emulsion by fluidized bed coating technology.” LWT – Food Science and Technology 62, no. 1 (2015): 187-93. doi:10.1016/j.lwt.2014.12.036.
Fonteles, Thatyane Vidal, Ana Karoline Ferreira Leite, Ana Raquel Araújo Silva, Alessandra Pinheiro Góes Carneiro, Emilio De Castro Miguel, Benildo Sousa Cavada, Fabiano André Narciso Fernandes, and Sueli Rodrigues. “Ultrasound processing to enhance drying of cashew apple bagasse puree: Influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds.” Ultrasonics Sonochemistry 31 (2016): 237-49. doi:10.1016/j.ultsonch.2016.01.003.
Hao, Fayi, Lixin Lu, and Jun Wang. “Finite Element Analysis of Moisture Migration of Multicomponent Foods During Storage.” Journal of Food Process Engineering 40, no. 1 (2016). doi:10.1111/jfpe.12319.
Hao, Fayi, Lixin Lu, and Jun Wang. “Finite Element Simulation of Shelf Life Prediction of Moisture-Sensitive Crackers in Permeable Packaging under Different Storage Conditions.” Journal of Food Processing and Preservation 40, no. 1 (2015): 37-47. doi:10.1111/jfpp.12581.
Kuang, Pengqun, Hongchao Zhang, Poonam R. Bajaj, Qipeng Yuan, Juming Tang, Shulin Chen, and Shyam S. Sablani. “Physicochemical Properties and Storage Stability of Lutein Microcapsules Prepared with Maltodextrins and Sucrose by Spray Drying.” Journal of Food Science 80, no. 2 (2015). doi:10.1111/1750-3841.12776.
Liu, Wei, Haifeng Wang, Xifeng Gu, Can Quan, and Xinhua Dai. “Certification of reference materials of sodium tartrate dihydrate and potassium citric monohydrate for water content.” Anal. Methods 8, no. 13 (2016): 2845-851. doi:10.1039/c5ay03067f.
Marquez-Rios, E., V.m. Ocaño-Higuera, A.n. Maeda-Martínez, M.e. Lugo-Sánchez, M.g. Carvallo-Ruiz, and R. Pacheco-Aguilar. “Citric acid as pretreatment in drying of Pacific Lion’s Paw Scallop (Nodipecten subnodosus) meats.” Food Chemistry 112, no. 3 (2009): 599-603. doi:10.1016/j.foodchem.2008.06.015.
Penner, Elizabeth A., and Shelly J. Schmidt. “Comparison between moisture sorption isotherms obtained using the new Vapor Sorption Analyzer and those obtained using the standard saturated salt slurry method.” Journal of Food Measurement and Characterization7, no. 4 (2013): 185-93. doi:10.1007/s11694-013-9154-3.
Rao, Qinchun, Mary Catherine Fisher, Mufan Guo, and Theodore P. Labuza. “Storage Stability of a Commercial Hen Egg Yolk Powder in Dry and Intermediate-Moisture Food Matrices.” Journal of Agricultural and Food Chemistry 61, no. 36 (2013): 8676-686. doi:10.1021/jf402631y.
Rao, Qinchun, Andre Klaassen Kamdar, Mufan Guo, and Theodore P. Labuza. “Effect of bovine casein and its hydrolysates on hardening in protein dough model systems during storage.” Food Control 60 (2016): 621-28. doi:10.1016/j.foodcont.2015.09.007.
Syamaladevi, Roopesh M., Ravi Kiran Tadapaneni, Jie Xu, Rossana Villa-Rojas, Juming Tang, Brady Carter, Shyam Sablani, and Bradley Marks. “Water activity change at elevated temperatures and thermal resistance of Salmonella in all purpose wheat flour and peanut butter.” Food Research International 81 (2016): 163-70. doi:10.1016/j.foodres.2016.01.008.
Wei, Meilin, Xiaoxiang Wang, Jingjing Sun, and Xianying Duan. “A 3D POM–MOF composite based on Ni(ΙΙ) ion and 2,2′-bipyridyl-3,3′-dicarboxylic acid: Crystal structure and proton conductivity.” Journal of Solid State Chemistry 202 (2013): 200-06. doi:10.1016/j.jssc.2013.03.041.
Wei ML, Sun JJ, Wang XJ “Preparation and proton conductivity evaluation of a silicate gel composite doped with a metal–Schiff-base–POM-MOF.”Journal of Sol-Gel Science and Technology 71, no. 2 (2014): 324-28. doi:10.1007/s10971-014-3370-0.
Yuan, X., Carter, B.P. and Schmidt, S.J. 2011. Determining the Critical Relative Humidity at which the Glassy to Rubbery Transition occurs in Polydextrose using an Automatic Water Vapor Sorption Instrument. Journal of Food Science,76(1): E78-89.
Zabalaga, Rosa F., Carla I.a. La Fuente, and Carmen C. Tadini. “Experimental determination of thermophysical properties of unripe banana slices (Musa cavendishii) during convective drying.” Journal of Food Engineering 187 (2016): 62-69. doi:10.1016/j.jfoodeng.2016.04.020.
Appendix:
Water Activity vs. Moisture Content
Water activity is often thought to be a more complicated measurement than moisture content. But making accurate, repeatable moisture content measurements is not as simple as it seems.
In theory, moisture content measurement is easy. Simply determine the amount of water in a product, and compare that to the weight of everything else in the product. In fact, it is actually a difficult and complex process to obtain an accurate percentage of water in a product. Here’s why.
Different reporting methods cause confusion
Moisture content is reported on either a wet or a dry basis. For the wet basis, the amount of water is divided by the total weight of the sample (solids plus moisture). For the dry basis, the amount of water is divided by the dry weight (solids only). Unfortunately, moisture content is often reported only as a percentage, without any indication of which method was used. Though it is easy to convert between wet and dry basis, confusion and potential problems occur when comparisons are made between moisture contents reported on a different basis. In addition, moisture content reported on a dry basis can actually result in a percentage value greater than 100%, causing more confusion.
Diverse measurement methods make comparisons impossible
The AOAC lists 35 different methods for measuring moisture content. These are classified as either direct or indirect measurement methods. Direct methods involve removing the water from the product (by drying, distillation, extraction, etc.) then measuring the amount of water by weighing or titrating. Direct methods provide the most reliable results but are usually labor intensive and time consuming. Some examples include air oven-drying, vacuum oven-drying, freeze-drying, distillation, Karl Fischer, thermogravimetric analysis, chemical desiccation, and gas chromatography.
Indirect methods do not remove the water from the sample. Instead, they involve measuring some property of the food that changes as moisture content changes. These methods require calibration to a primary or direct method. Their accuracy is limited by the accuracy of the primary method. Indirect methods are usually fast and require little sample preparation but are less reliable than direct measurement methods. Examples of indirect measurement methods include refractometry, IR absorption, NIR absorption, microwave adsorption, dielectric capacitance, conductivity, and ultrasonic absorption.
Further complicating the process of measuring moisture content is that one measurement method does not necessarily provide the same results as another, and the measurement method is normally not reported with the moisture content value.
Even direct measurement methods do not provide consistent results. Any method that requires heating the sample (i.e., loss-on drying) can lead to the loss of organic volatiles or decomposition of the sample (especially for samples containing high levels of sugar). For example, if organic volatiles are present in a sample or if the sample decomposes while being dried, a Karl Fischer analysis, which is not susceptible to volatile loss or decomposition, will give different results than a loss-on drying analysis.
Variability is difficult to avoid
One answer to these problems is to simply use a consistent method and only compare values that have been obtained in the same way. Unfortunately, consistency in measurement method for moisture content analysis still will not eliminate all problems. Consider, for instance, loss-on drying. This method seems simple enough. A sample is weighed, and the weight is recorded. The sample is then transferred to an oven, allowed to dry, and the dry weight is measured. The amount of water is determined by subtracting the dry weight from the initial weight, and the moisture content is then calculated as the amount of water divided by the dry weight or total weight, depending on the reporting method.
Even this simple loss-on-drying method is mined with potential variability traps. The most fundamental is that the term ‘dry’ has no real scientific meaning and has never been well defined. Instead, an arbitrary dryness that is reproducible has to be established for each sample. “Dryness” is often defined as the point at which weight loss ends. However, thermogravimetric graphs show that weight loss levels off at different temperatures for different products. Also, depending on the product, the length of time needed to achieve “dryness” will differ, and a temperature which produces “dryness” in one product may cause decomposition in another. This means that each sample has a unique ideal oven temperature and drying time. This ideal time/temperature combination is available in the literature for some products, but there are many for which it is not available. It is difficult to know which combination to use for untested products. If the same time/temperature combination is not used, the resulting moisture contents should not be compared.
Another complication is that many ovens set at one temperature can vary over time from that temperature by as much as 15 °C, and two ovens set to the same temperature can vary by as much as 40 °C.
Additional sources of variation for just the loss-on drying method include: oven vapor pressure, sample preparation methods, sample particle size, sample weighing, and post-drying treatment. It is interesting that despite the potential pitfalls, when a loss-on drying moisture content is reported in literature, it is immediately accepted as correct. In addition, when comparisons are made between moisture content methods and one of those methods is loss-on drying, it is always assumed that the loss-on drying measurement is correct.
What is “dry”?
Defining “dry” would be helpful in eliminating some of the inconsistency associated with moisture measurement. The best way to define dry would be to identify an oven-dry water activity. Then, the dry weight would be the weight of the sample when it has achieved this oven-dry water activity. Under common ambient conditions of 25 °C and 30% RH, an oven set to 95 °C would create an oven-dry water activity of 0.01 aw inside the oven, assuming that the vapor pressure in the oven is the same as the air. An oven that maintained conditions where its oven-dry water activity was always 0.01 aw, regardless of ambient conditions, would create a scientifically “dry” condition. In this type of oven, any product could be declared dry when its weight stopped changing. Its water activity would be 0.01 aw, and its weight would be the dry weight. The vapor pressure and temperature of the oven could be adjusted to prevent release of volatiles as well, as long as the water activity in the oven was maintained at 0.01 aw. Using this method would eliminate the inconsistency that results from multiple measurement methods and an unclear definition of “dry.”
A more accurate moisture analysis
Moisture content provides valuable information about yield and quantity, making it important from a financial standpoint. It also provides information about texture, since increasing levels of moisture provide mobility and lower the glass transition temperature. But obtaining correct and consistent moisture content values can be difficult, and a moisture content measurement cannot be taken at face value without information about the methods used to generate it. Additional problems arise when the amount of water in a product is used to tell a story it doesn’t really tell, involving product consistency, quality, or microbial safety. In these and other cases, water activity is the more accurate measurement. For a complete moisture analysis, food and pharmaceutical developers should measure both water content and water activity. In addition, moisture sorption isotherms may be used to pinpoint where optimal shelf life, texture, safety, and quality can be achieved and maintained.
A scientific definition of water activity
Water activity is derived from fundamental principles of thermodynamics and physical chemistry. As a thermodynamic principle there are requirements in defining water activity that must be met. These requirements are: pure water (aw = 1.0) is the standard state, the system is in equilibrium, and the temperature is defined.
In the equilibrium state
μ = μo +RT ln (f/fo)
where: μ (J mol-1) is the chemical potential of the system i.e., thermodynamic activity or energy per mole of substance; μo is the chemical potential of the pure material at the temperature T (°K); R is the gas constant (8.314 J mol-1 K-1) ; f is the fugacity or the escaping tendency of a substance; and fo is escaping tendency of pure material (van den Berg and Bruin, 1981). The activity of a species is defined as a = f/fo. When dealing with water, a subscript is designated for the substance
aw = f/fo
aw is activity of water, or the escaping tendency of water in system divided by the escaping tendency of pure water with no radius of curvature. For practical purposes, under most conditions in which foods are found, the fugacity is closely approximated by the vapor pressure (f ≈ p) so
aw = f/fo ≅ p/po
Equilibrium is obtained in a system when μ is the same everywhere in the system. Equilibrium between the liquid and the vapor phases implies that μ is the same in both phases. It is this fact that allows the measurement of the vapor phase to determine the water activity of the sample.
Water activity is defined as the ratio of the vapor pressure of water in a material (p) to the vapor pressure of pure water (po) at the same temperature. Relative humidity of air is defined as the ratio of the vapor pressure of air to its saturation vapor pressure. When vapor and temperature equilibrium are obtained, the water activity of the sample is equal to the relative humidity of air surrounding the sample in a sealed measurement chamber. Multiplication of water activity by 100 gives the equilibrium relative humidity (ERH) in percent.
aw = p/po = ERH (%) / 100
Water activity is a measure of the energy status of the water in a system. There are several factors that control water activity in a system:
- Colligative effects of dissolved species (e.g., salt or sugar) interact with water through dipole-dipole, ionic, and hydrogen bonds
- Capillary effect, where the vapor pressure of water above a curved liquid meniscus is less than that of pure water because of changes in the hydrogen bonding between water molecules
- Surface interactions, in which water interacts directly with chemical groups on undissolved ingredients (e.g., starches and proteins) through dipole-dipole forces, ionic bonds (HO or OH), van der Waals forces (hydrophobic bonds), and hydrogen bonds3–
It is a combination of these three factors in a food product that reduces the energy of the water and thus reduces the relative humidity as compared to pure water. These factors can be grouped under two broad categories: osmotic and matric effects.
Due to varying degrees of osmotic and matric interactions, water activity describes the continuum of energy states of the water in a system. The water appears “bound” by forces to varying degrees. This is a continuum of energy states rather than a static “boundness”. Water activity is sometimes defined as “free”, “bound”, or “available water” in a system. Although these terms are easier to conceptualize, they fail to adequately define all aspects of the concept of water activity.
Water activity is temperature dependent. Temperature changes water activity due to changes in water binding, dissociation of water, solubility of solutes in water, or the state of the matrix. Although solubility of solutes can be a controlling factor, control is usually from the state of the matrix. Since the state of the matrix (glassy vs. rubbery state) is dependent on temperature, one should not be surprised that temperature affects the water activity of the food. The effect of temperature on the water activity of a food is product specific. Some products increase water activity with increasing temperature, others decrease aw with increasing temperature, while most high moisture foods have negligible change with temperature. One can therefore not predict even the direction of the change of water activity with temperature, since it depends on how temperature affects the factors that control water activity in the food.
As a potential energy measurement, it is a driving force for water movement from regions of high water activity to regions of low water activity. Examples of this dynamic property of water activity are: moisture migration in multi-domain foods (e.g., cracker-cheese sandwich), the movement of water from soil to the leaves of plants, and cell turgor pressure. Since microbial cells are high concentrations of solute surrounded by semi-permeable membranes, the osmotic effect on the free energy of the water is important for determining microbial water relations and therefore their growth rates.
A powerful measurement for QA/QC and formulation
Water activity is a thermodynamic measure of the energy of water in a product. Why should your company learn how to measure water activity? It is directly related to the microbial susceptibility of food products. It also has direct relationships with many of the reactions that end shelf life in foods. Because it is measured on a scale with a known standard, it is particularly well suited to being a safety and quality specification.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
