Expertise Library
What is a moisture analyzer? How do balances, ovens, titrations and NIR compare?

A moisture analyzer determines the moisture content of a sample. There are several types of moisture content (Mc) instruments. Traditional moisture analyzers use the loss loss on drying method and are often referred to as moisture balance or moisture meter. In 2022, advancing technology will enable a variety of new moisture measurement methods and each method has its strengths and weaknesses. Let’s break it down.
Water is almost everywhere, and often, it’s crucial to controlling texture, quality, safety and other characteristics of manufactured goods.
In industries where products are sold by weight, misunderstanding moisture content can lead to giving away millions of dollars worth of product or causing catastrophic product failures.
As such, many manufacturers – of everything from food to lumber – need to know how much moisture is in any given substance. In addition, many manufacturers, especially in the food, pharmaceutical, and cannabis industries, are compelled by government regulations to measure moisture content.
Enter moisture analyzers: instruments that calculate how much water there is in a material by testing a small sample.
Methods vs instruments
There are several classes of moisture content-measuring instruments. Each class uses a different method of measurement, and each method has strengths and weaknesses.
Thermogravimetry, sometimes called loss-on drying (short for “measurement of mass loss on drying”) is the most common and well known method. Thermogravimetry involves heating a sample, then deducing the amount of moisture in it by comparing its pre-drying mass to its post-drying mass. Thermogravimetry assumes that water is only substance that will evaporate during drying, though this isn’t always the case.
Other methods deduce moisture content by testing how a sample responds to stimuli like light or electricity, then using the result to deduce moisture content.
Choose moisture content methods and analyzers carefully
In theory, measuring moisture content seems simple – weigh it, dry it, weigh it again, and the difference between both weights tells you how much moisture there was, right?
Unfortunately, no. Sometimes the water in a substance is so tightly bound to the rest of its makeup that when you try to extract only water, other volatile materials evaporate too. It’s almost impossible to differentiate between water lost and other volatiles lost.
In other situations, efforts to extract moisture (usually at high temperatures) can change the sample’s chemical composition, literally creating more water in the sample.
If you decide not to use thermogravimetry in hopes of avoiding those problems, you’ll soon encounter the toxic chemicals used in titration and the calibration drift of NIR. And that’s just the beginning – there’s more in store.
Getting a truly precise, scientifically reliable moisture content measurement can be tough, but it isn’t impossible, so long as you understand your sample material and choose the method and instrument to match.
Below, you’ll find a quick primer on each class of instruments and how they can best be used.
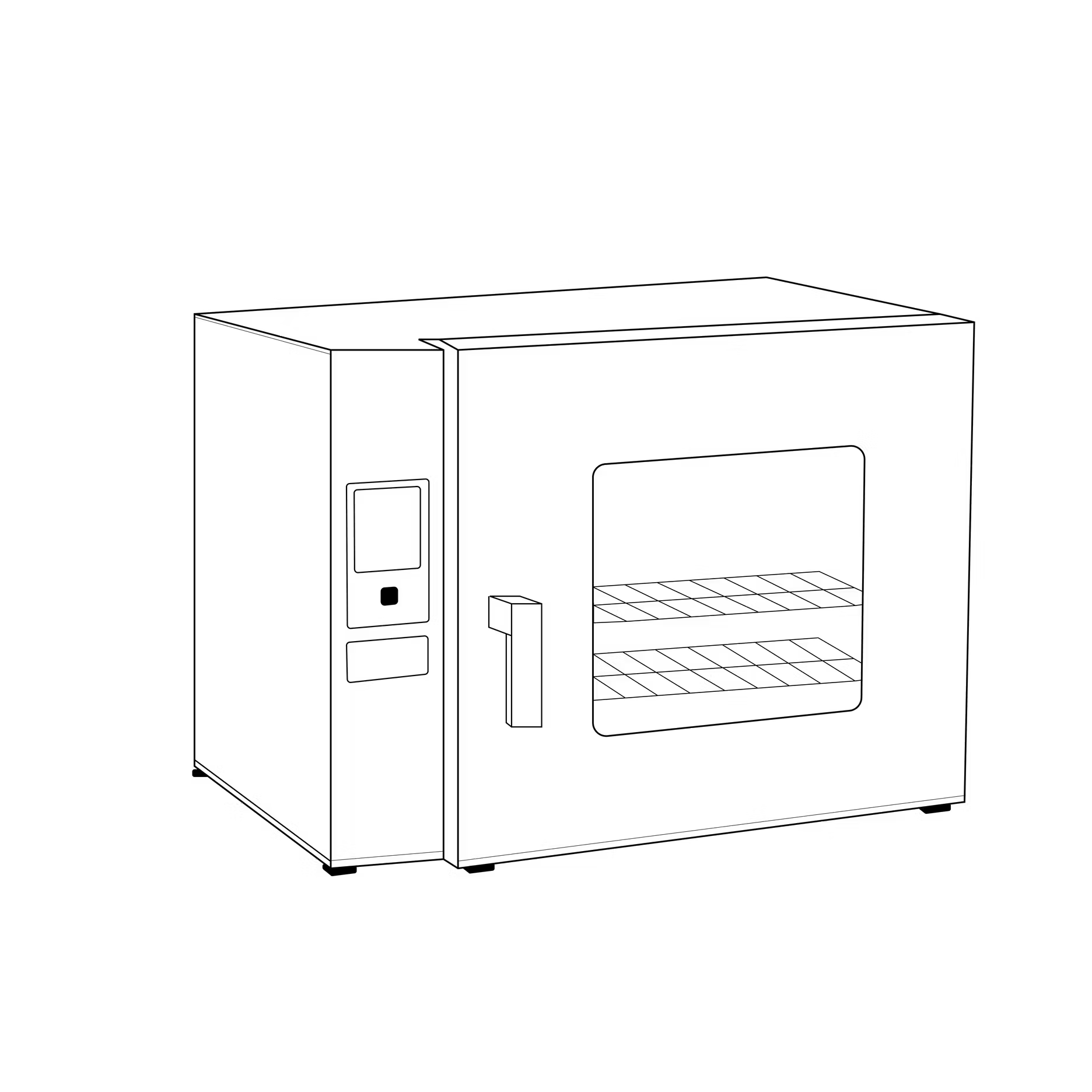
(caption: A line drawing of a drying oven used for moisture analysis and moisture content determination in food science labs)
Drying ovens
Thermogravimetric moisture analysis using a drying oven is the most traditional way to measure water content, and remains the official reference method for many regulatory bodies, including the AOAC and USP.
However, it’s also the most manual and time-consuming method – the user is the real “moisture analyzer” in this case, the oven is simply a heat source. Official AOAC methods (which are typical of most official methods) require variations of the steps below, depending on sample type:
- Dry an empty sample container at a specified temperature for three hours
- Transfer the container to a desiccator to cool
- After the dish is cool, pour in and spread a specified amount of sample
- Place the dish and sample back into the oven at a specified temperature for several hours
- Remove the dried sample and dish and place in a desiccator to cool
- After cooling, re-weigh the dish and sample
- Calculate moisture content with a specified equation
Ovens are ubiquitous in food science and quality control labs. They require attention to detail and plenty of hands-on time but can deliver precise, reference-quality results when used correctly. And unlike many of the methods below, ovens allow users to dry many samples at once – though you’ll still be required to cool and weigh all those samples separately.
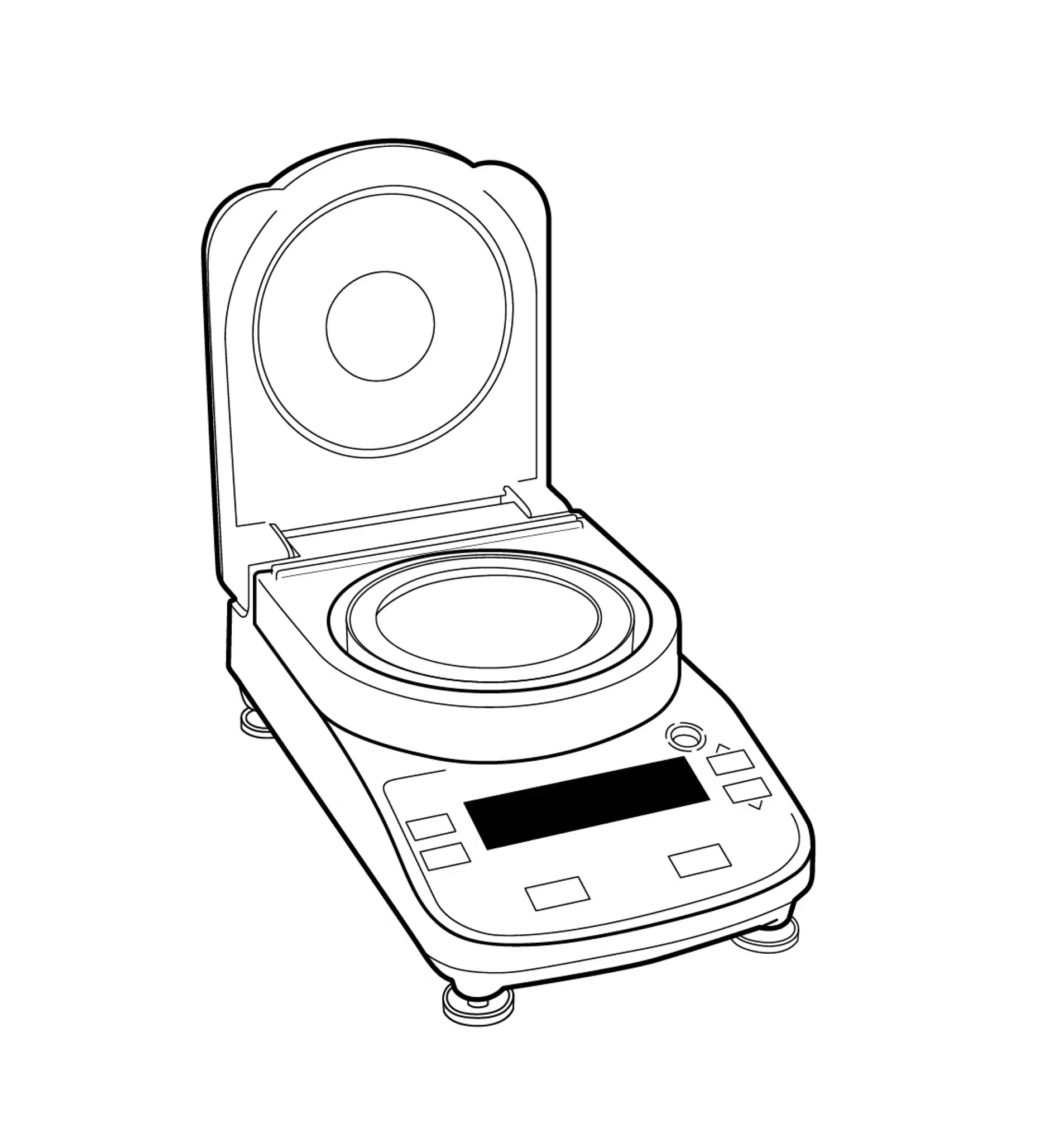
Moisture balances
Moisture balance analyzers apply the same thermogravimetric principles that drying ovens do, but they automate weighing and only measure one sample at a time.
Instead of keeping the heat source and weighing mechanism separate, moisture balances integrate a scale (or balance), a space for a sample, and a heater (usually a halogen bulb), all in one instrument. After the user inserts a sample and chooses a drying program, the instrument heats the sample at a set temperature and for a set amount of time.
Since the balance is built in, users don’t have to remove and weigh the sample over and over – the device does it automatically. The often-used halogen heat source reaches high temperatures quickly, and since moisture balances only have space for one sample, the small space heats quickly.
To avoid common pitfalls of moisture balances, choose your instrument carefully, and set your drying program deliberately. Temperature control is often an issue – aiming for quick dry times, many balances get too hot too soon and simply burn samples, creating a scorched shell that seals in remaining moisture, changes the chemical structure of the sample, and distorts final results (not to mention stinking up your lab).
Microwave moisture analyzers
A more recent entrant to the class, microwave moisture analyzers are yet another “thermogravimetric” or loss-on drying method. Rather than use infrared energy to heat samples, microwave moisture analyzers bombard samples with microwaves, heating the water in a sample and causing it to evaporate.
Microwave lab analyzers can heat samples very quickly, particularly high-moisture samples. As such, they’re popular for measuring liquids and pastes such as yogurt, cheese and dairy products.
Because they focus on liquid and pasty substances, preparing any sample that isn’t already liquid usually requires grinding it to a paste and smearing it on a pad before testing – a process that can inadvertently change the sample’s moisture content.
Microwave analyzers don’t excel at low moisture content samples, as they tend to scorch and burn.
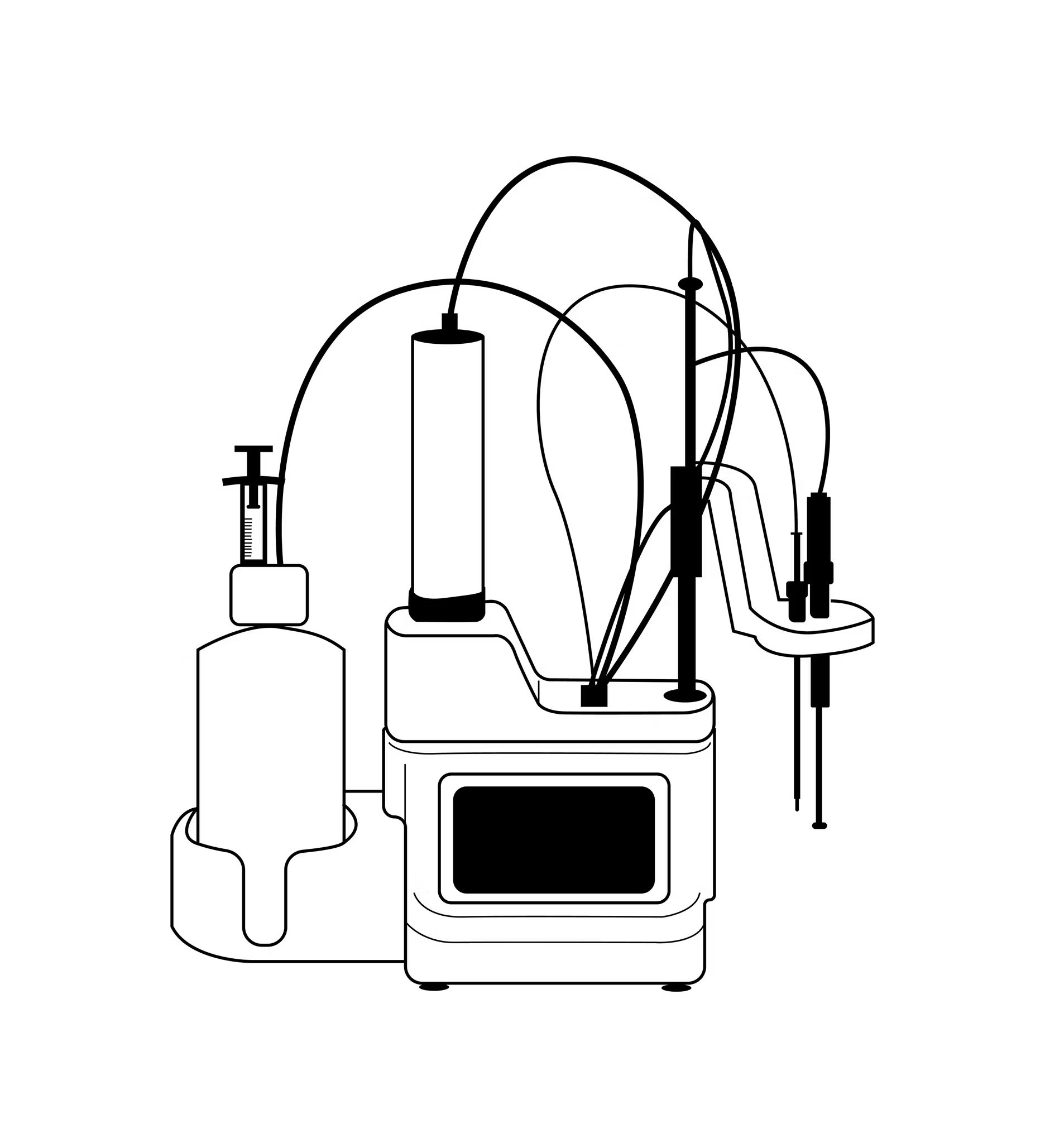
Titrators
Karl Fischer titration, named for the scientist who invented it in 1935, measures infinitesimally small amounts of moisture. In most cases, it’s the most precise method of measuring moisture content, so it’s often used in the pharmaceutical and petroleum industries, where any trace of moisture can have an outsized impact.
Titration is an involved process. Karl Fischer moisture analyzers work by mixing your sample with a chemical that reacts with water. After the reaction, the titrator can quantify the amount of water with an electrode. Titration can take anywhere from a few minutes to more than 30, depending on how compatible the sample is with the necessary solvents and if workarounds are needed.
Titration’s precision comes at a cost. One-button titrators have significantly simplified the process, but a comparatively extensive understanding of chemistry and lab science is still needed to set tests up correctly, choose appropriate reagents, handle dangerous chemicals, and interpret the results.
Near-infrared Spectroscopy (NIR)
NIR is an indirect method of measuring moisture content that doesn’t require drying, weighing, mixing chemicals, or even touching the sample at all.
NIR works by blasting samples with light within a specific spectrum. Because certain molecules interact with the light in different ways, by measuring how much of the light the sample reflects back, assumptions can be made about how much moisture it contains.
Because NIR can make readings quickly and without touching or affecting samples at all, it’s often used on production lines to get quick moisture readings during processing.
NIR is an indirect method, so it requires regular calibration to a reference method – which often ends up being one of the other measurement methods listed above. NIR instruments’ readings typically maintain variance around 1.5 or less standard deviations of the reference method used to calibrate it.
Choosing the right analyzer
There are only a few reliable methods of moisture analysis, but there are countless moisture analyzers on the market. When choosing an instrument, first determine which method – titration, NIR, or thermogravimetry – is right for you, then move on to choosing a specific instrument.
Even within the constraints of a single method, analyzers come in all shapes and sizes. Some analyzers emphasize speed over precision, others measure more than just moisture content, and a few can automate measuring many samples at once.
Consider what specific features you need from your analyzer, then make your choice based on your application.
Get the complete picture
Learn everything about how better moisture content measurements can optimize your yields, increase your profit and improve product quality — all in one place.
Written with the cannabis, food manufacturing, and pharmaceutical industries in mind.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
