Webinars
Natural ingredients 101: Moisture in dried fruit & nuts
Natural ingredients can be unreliable and unpredictable. But skyrocketing demand for clean labels and familiar ingredients can’t be ignored – so what’s a food manufacturer to do?
Measuring and managing the moisture in natural ingredients is one of the most reliable and cost-effective ways to prevent the formulation challenges, production delays, and quality concerns they often cause.
The METER Food R&D Lab has helped some of the world’s largest food companies understand and eliminate the inconsistency in their natural ingredients. In this broadcast, Mary Galloway (R&D lab manager) and Zachary Cartwright (lead food scientist) will share how they helped and what they learned in the process.
Transcript, edited for clarity
Dr. Zachary Cartwright: Welcome to Natural Ingredients 101, where we'll be talking about dried fruits and nuts. Whether you're using these as final products or as ingredients, we're here to talk about how you can control and monitor the water in these types of products.
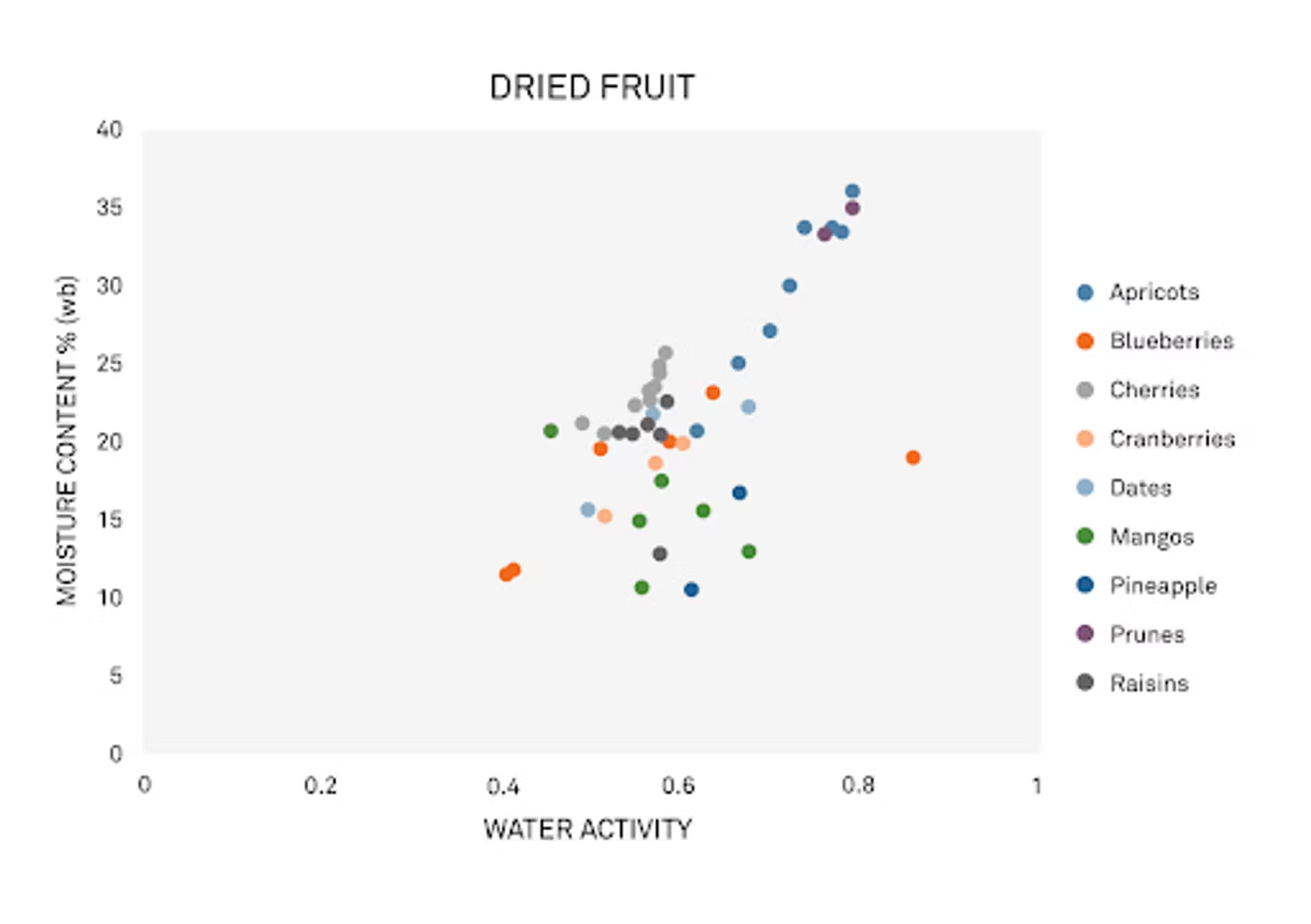
First, we're going to talk about the variability that exists in dried fruits and nuts. We're going to bring up an image here, starting with fruit. It looks like a scatter plot showing the relationship between water activity on the X-axis, and moisture content on the Y. This is data that Mary collected – I'll hand this to you, Mary. Maybe you can talk about what we're looking at here.
Mary Galloway: Sure. There's a lot of variability within different types of fruit, but there's also quite a bit of variety within one type of fruit. Let’s pull out the data for blueberries.
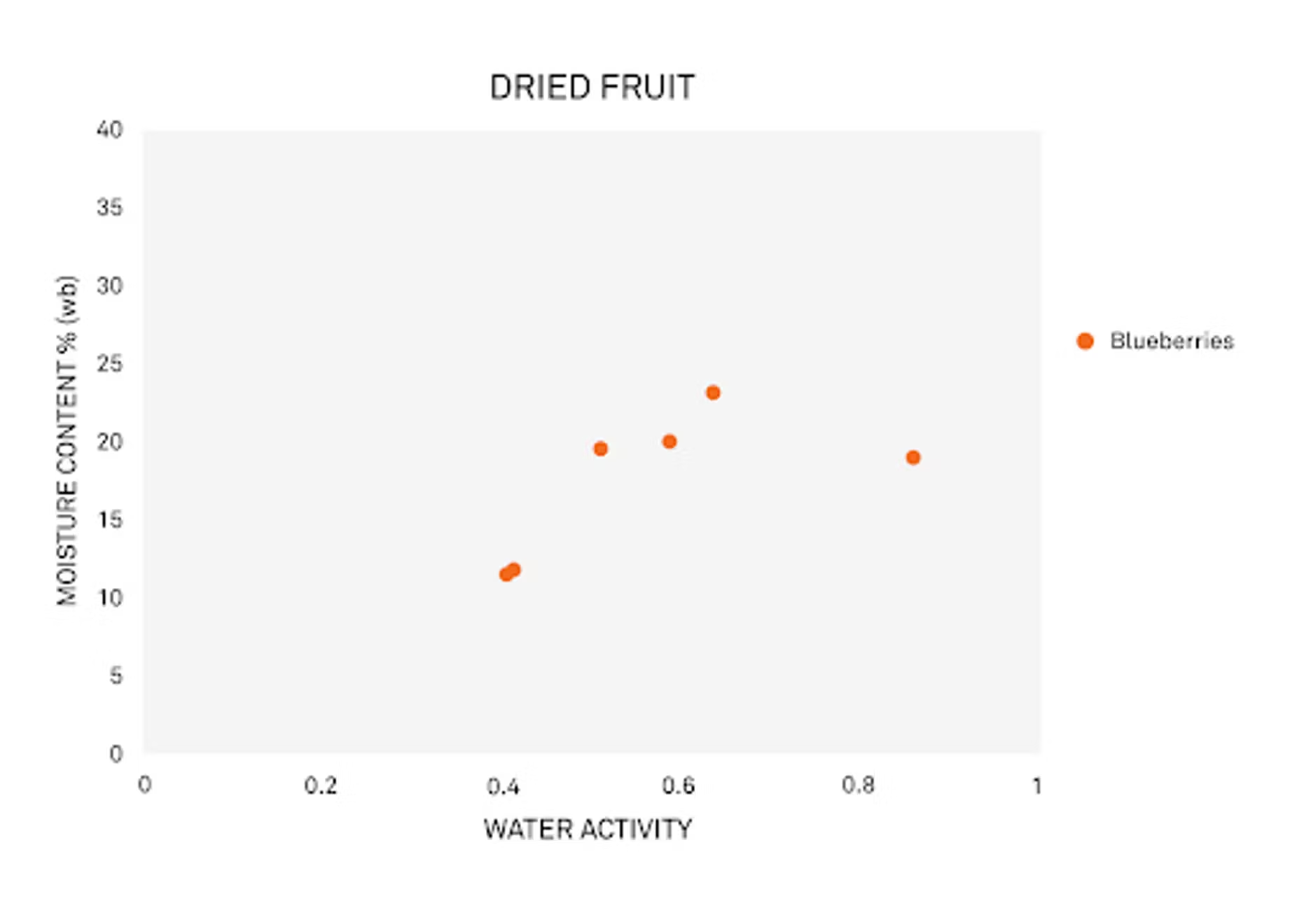
We're going to use blueberries and almonds as our examples in this webinar, but the principles apply to other fruits and nuts as well.
Some of the variability you'll see in the fruit has to do with the sugar and fiber content that you'll find in the fruit. Like I mentioned, these factors can vary between the different kinds of fruit. For example, mangoes – they're a very fibrous fruit, and you get a really different relationship there. We've got a lot of variability within water activity and moisture content for that. With blueberries, there’s more of a predictable relationship between water activity and moisture content.
But even then, you'll get differences in the sugar and fiber content even in blueberries. Varietal differences play an impact, so does the drying method and how it was processed. Was it processed as a natural, unsweetened blueberry, or is there sugar or something else added? Even the growing season can have a big impact. Even if you have exactly the same grower and the same variety year after year, you can have differences in the characteristics of the berry because of the variations in growing season.
Another factor that you'll see variations in is pH. pH tends to impact fruit in particular, more than other ingredients. Fruit tends to be acidic, which means it has a low pH. When you have something that has more acidity, you can get away with a little bit higher water activity, since you won't have as much trouble with mold limits.
Say you’re producing a bar or snack, and you get supplied with a fruit at a specific pH. You find the water activity limit that’ll keep it safe, then move on. But maybe the next year, the fruit ships at a lower pH – now you don't have that safety buffer.
ZC: I'm glad you brought up the growing season. I've worked with a lot of companies, and even though they produce dried cherries or the same product year after year after year, they still vary by growing season. That makes measuring moisture a challenge even from year to year.
What about nuts? What type of variability do you see with nuts? More variation or less? Or is it a similar scatter plot that we're going to look at?
MG: Still a scatter plot, but not as much. We have a graphic here where we're showing a variety of nuts from a similar study that we did. We have a lot of different nuts, almonds, peanuts, cashews, and you can see it is a little crazy.
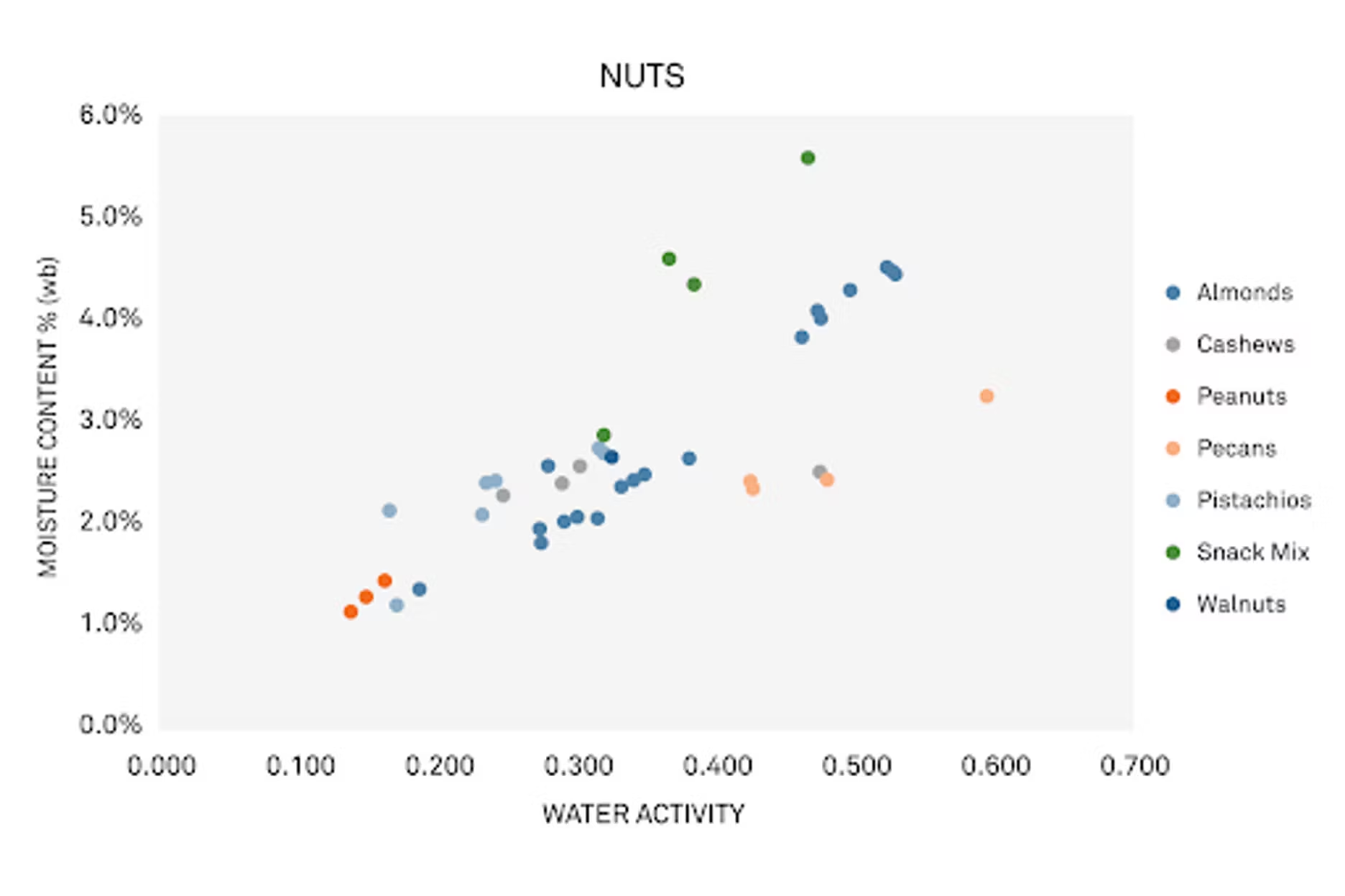
When we pull out almonds specifically, you can see that there's a pretty nice trend, but it's different from the blueberries. We're in a much lower water activity range. You don't see as much variability, but there is still variation, and for similar reasons.
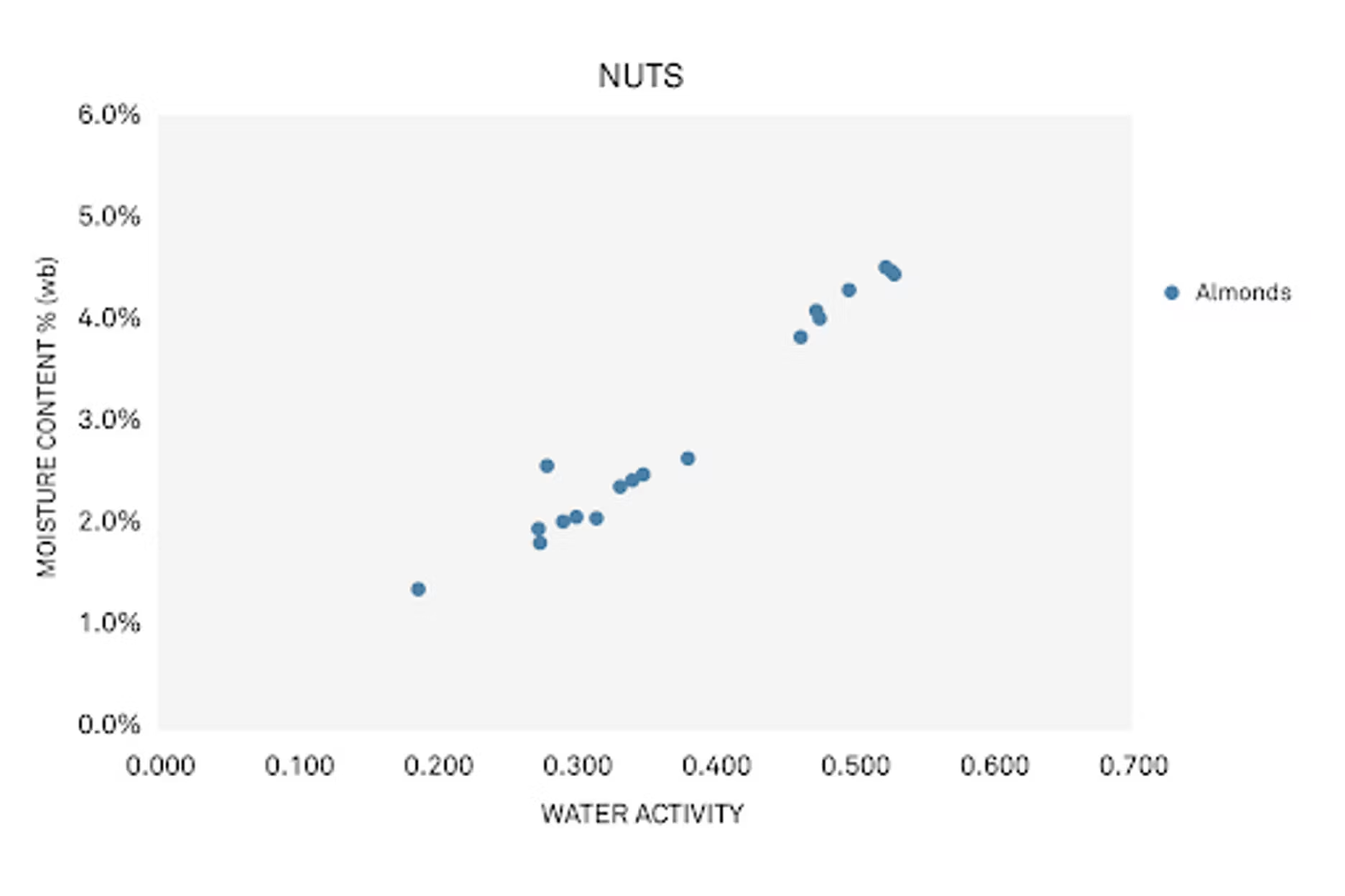
But here, even beyond growing season and what we talked about earlier, processing can be an important factor. Is it raw? Is it dried? Is it salted? Does it have some flavoring? Those all impact the water activity and moisture content of a nut.
Also, something to consider is that nuts live in a lower water activity range. When they're low, they can be susceptible to rancidity. We've talked about that in other webinars.
Basically, when you have low water activity, lipids are more exposed to the environment and oxidize more. That's a consideration for both nut growers and importers.
We can use the isotherm relationship to visualize it. You can see a definite relationship between the water activity and moisture content.
But like with fruits, you have to consider what you'll use your nut for, and in what form. They have less variability, which is great, but are we using it as a snack, are we using it as an ingredient? Is it whole, salted, is it a paste, is it just pieces? Those all impact how easy it is to change water activity and moisture content for the nuts.
ZC: Great. To summarize this first section, what we're trying to demonstrate here is that there's a lot of variability, whether it's fruit or nuts. We've shown these two scatter plots. We're going to focus the next section on the implications of variability, how to reduce variability, and then we'll move into isotherms and finish with what happens when we mix these things together.
Implications of variability
ZC: Let’s talk about some of the implications of variability. We're going to start with the obvious: texture and taste. Mary has set up a small experiment for us. I'm going to let her describe what we have here in front of us.
MG: Yeah. We set two different conditions to demonstrate the differences between an over-hydrated or a hydrated fruit or nut versus something that's been over-dried. So these blueberries here and these almonds, we've equilibrated them to a higher water activity. They're at about 0.7.
I know it's a little hard to see on the video, but for a blueberry, that’s soft. There's a really soft texture to it. That’s not terrible for a blueberry, although you do want to be careful because there is that mold limit at 0.7. Even with that pH that I talked about, I would not recommend getting too close to that zone. This texture is soft enough to be ideal if you were just eating these by themselves – as long as they weren't moldy.
But for the almond, this water activity level is different. This is too moist, too high water activity for the almond, and it's going to be soft. Imagine what it's like to bite into a soft nut. As I'm pushing on it, I can feel it give a bit. Everybody knows what that's like – it’s not good.
In comparison to the more moist samples, here we've got dried almonds and blueberries. For the almond, that's a natural place for it to be, at a lower water activity. When you bite that, you get a nice crunch. But for the blueberries, it's way too hard. This is not a happy place for the blueberries to live.
Just like a soft nut is gross, a rock hard fruit isn’t appetizing. Listen – I can rattle these around in the jar. It's noisy.
ZC: Yeah. You can hear the difference. Definitely.
MG: You hear that these are dried out, right? When we're looking at trying to find a balance between water activity and moisture content, you need to consider what that's going to do to your ingredients. Are you going too low and we're getting a hard texture, or are you going too high in water activity and getting a soft texture? We need to find the balance between the two.
ZC: I just want to ask you a little bit about how you set this experiment up. How did you determine what water activities to equilibrate to and where do those numbers come from?
MG: Mostly we look at the native water activity of our ingredients. We do have some examples where we've taken these as they are naturally and blended them. You could see where these are going to, if we didn't do anything to them, if we just took them straight from a supplier and blended them, where are they going to end up?
We start where they natively begin and go from there. There are definite reasons and some challenges when we have low water activity, like for the nuts about the rancidity, wanting to stay away from that. But mostly we just stayed in their natural areas and then flipped them – the low water activity for the almonds, we put the blueberries down there and vice versa for the upper for more of a moist blueberry and to see what it was going to do with the almonds.
ZC: This also goes back to our first section where we talked about considering whether you’ll use this as an ingredient or as a final product. Because you might want a drier blueberry in a cereal or something like that. But if you're snacking on the blueberry, then you'd definitely want something softer. Just thinking about how these are going to be used will help you to set your target.
Measuring and account for moisture variability in fruits and nuts
ZC: Next, we're going to talk about measuring and accounting for variability in fruits and nuts. Mary, I'd like to ask you, how is this done now? How do people measure moisture in fruits and nuts?
MG: Yeah, mostly it's just moisture content that they're using. Both of these industries have been around a long time, so they have some archaic ways to determine moisture content.
Often loss on drying is the measurement method, sometimes a moisture balance, which means you're getting a faster moisture content, but you have a really hot lamp that is going to try to drive off the moisture. The problem with this is that both fruits and nuts have issues with high temperatures. Fruit has high sugar, so you can get browning and some hydrocarbon conversion. Then the nuts can toast, and that can throw off moisture content.
The fruit industry actually also has some other ways, older ways where they're measuring a change in electro conductivity of a fruit paste. Not the most accurate way to do it, but it's traditional.
When you have traditional data, it's hard to pull away from using that measurement when that's sure where all your historical data has come from in the past. But this might be a really great transition to talk about the relationship between moisture content and water activity and why that's important.
ZC: Yeah. In the past sections, we've mentioned water activity quite a few times. The reason for that is water activity is a much easier and more precise way to think about the water in your product and how it directly relates to quality or even safety. So what we're going to show here is an image that shows the relationship between water activity on your X-axis and moisture content on your Y. We showed this earlier when we showed those scatter plots. But looking at this a little bit closer will help to understand how this relationship can be used, especially as we talk about mixing in one of our next sections.
If we were to map the entire relationship between water activity and moisture content at a specific temperature, then we get this entire moisture map that we call a moisture sorption isotherm. We have a really unique way to do this at METER Group.
We have a method called the dynamic dewpoint isotherm. Basically what this allows us to do is create a really high resolution isotherm so that we can see that entire relationship. The one way that this is really useful is that as you move up and down this curve, it can help us to understand things like reaction rates. You mentioned it earlier, things like lipid oxidation will occur at certain water activities. If we use this moisture map, then we can set the right target for our almonds, around 0.3 water activity. There are also browning reactions or enzyme activity, other things that can happen as we move along this curve. We can also use the isotherm to predict when microbial challenges may present themselves. Whether it's mold or bacteria or yeast, these things are going to start to occur as we go up in water activity. That's something that you can keep in mind when you look at this isotherm curve.
The last thing to keep in mind are texture changes. If we were talking about a powder, it might be a kicking and clumping point. Even here where we're talking about texture, these texture changes may show up on our isotherm curve and may be something that we can identify what we call a critical water activity. We know the range of water activity that we need to stay within before we start to have noticeable changes. The reason I bring up this curve is because it's a really good way to think about all of these different things affecting the safety and the quality of the product that we're looking at. That isotherm is going to be specific to each of the products or each of the ingredients that we look at. Is there anything that I'm missing, anything else that you would add about using isotherms?
MG: No, you've done a really good job and hopefully you'll be able to visualize, as Zach was talking there about the different influences that water activity can have on a product, because as we increase water activity, different changes can happen. There's different modes that are happening within there. Some of them may make perfect sense, like the browning reaction, it has to do with the water that's in the surrounding area of something. It's like switching different modes as we're moving up in water activity, but that all has an impact on a product that's physics and you need to be aware of.
ZC: Let's say that we have an isotherm now, how can that isotherm be used to improve precision, especially if you think of it, I believe we have an example with the pecan.
Maybe you can walk through that example and talk about how having variation in your moisture content measurement is going to show up on the isotherm curve.
MG: This is where precision in the measurement really matters. Because if we're using an older, traditional way, and this was a real life example with pecan of the pecan grower in particular where their method to measure moisture content was only accurate to 0.5. just a half a percent more moisture content, which seems pretty good, right?
ZC: Yeah, really good, compared to what I normally see.
MG: Yeah. you're thinking, oh yeah, half a percent. I'd be happy with that. But when we look at that relationship with moisture in the pecan, we can see that, and we have a secondary graphic that's showing a plus or minus of 0.25. Half of that. if we say 0.5, it could be pushing it up a little or pushing it down. What does that mean in terms of safety and mold growth?
When we look at the isotherm, we'll notice that even if we just scooch it up a little bit, a quarter of a percent increase in moisture content actually puts us in that 0.7 water activity, which is where mold can start growing. That is a big deal. If you don't know, when you have this variability in your one measurement that you're using, when you really look at it's not telling you all that you need to know.
But water activity, the granularity in that measurement is huge. So you can see in the second graphic, we have a range of 0.01 water activity, and we can measure every little bit of that. So it's very easy to see where we're really going to lie in the moisture content, and really it's about water activity and not moisture content anyway, where that mold limit is going to happen or where we're going to have some issues in that realm.
ZC: Again, I just want to point out that 0.5%, which may not even be realistic – most of the time I see plus or minus one or even 2% – but this graphic does a really good job of showing that if you're close to that microbial limit and you're only measuring moisture content, then you have a really good likelihood that some of your product is going to have issues with mold.
The way that I see some companies try to deal with this is just across the board over-drying, but then you run the risk of messing with your yield and your revenue, but maybe over-drying and getting to the point of lipid oxidation occurring.
What we're trying to show here is that if you use water activity, that's the correct measurement, and you shouldn't just be measuring the final product, but you should be measuring incoming ingredients throughout production, and then also the final product. there's a whole range of places that you should be watching how your water is changing over time.
MG: Right. That's a really good point because, and we talked about it before, if you have a supplier that you continue to use year after year, they're still going to have variability, it's just unavoidable. You just need to know, and then you can make adjustments to your own process. Even if you have the same supplier, like I said, you're still going to get the variability. if you're measuring, as it's coming in, then you'll know what you're getting.
Water activity is just a better metric in the sense that that is what's going to be driving any microbial growth, it's not moisture content, and other things, moisture migration, which we will be talking about in the future. But it's an easy measurement. It's also faster than many moisture content methods.
ZC: Yeah. That's a perfect segue because in our next section, we will talk about mixing these things together and predicting moisture migration and how that can be done with an isotherm.
Moisture challenges when working with fruits and nuts
ZC: Next we're going to talk about one of the biggest challenges about fruits and nuts. That's when you start to mix these things together, and understand which way the moisture's going to move and the implications of making a mixture.
Mary, I'm going to hand this over to you since we have talked about isotherms. How can we use these isotherms to predict what will happen when we combine the blueberries and the almonds?
MG: A misconception is that moisture moves because of amount. When we're talking about that, we're talking about moisture content. If something has a higher moisture content, then it's going to be the one moving the moisture, and moisture's going to be moving out of that to the other things. it's actually not the amount, it's the energy level. When there's more energy, then we're going to have movement. It's physics.
If we look at our blueberries and our almonds here, and I'm going to take this example where our blueberries are at a 0.48, which is a pretty decent place for blueberries, meaning in terms of water activity. our almonds here are .30, which is a pretty good place too. It avoids any rancidity, but it's that nice texture that we talked about. What's going to happen is the blueberries being at higher water activity, the moisture is going to move down into or over to the almonds themselves or any other ingredient.
That's going to be the factor. Now we could lower the water activity of our blueberries by adding what we call a humectant, which is like a sugar or a salt. I mean, there's other things, other sweeteners, and that will lower the water activity. The reason I mention that is because if these are close to each other, we won't be having any moisture migration. Even though this could have a lot of moisture, a higher moisture content, if we can bring the water activity down where these are similar, we won't have any moisture moving at all. A good example of that is when you look and you mentioned earlier fruit in cereal, you have a cereal with a crispy flake that's low water activity, low moisture. We have a fruit, a raisin, which obviously has a lot of moisture and can be higher in water activity.
How does that work? How can you have them live in the same space? That's because there's sugar generally added. especially when you look on the outside of a raisin, that lowers the water activity so they're closer. That's some way to think about what we're talking about in terms of it's really the difference in water activity and not moisture content. We do have an example to demonstrate what it is like to predicatively model when we blend these things together? Because that is a big thing. They're fine on their own. Now when we're going to put them together, what are we going to get?
In our example here, we've got our two isotherms. The blueberries are in the blue trace, and then we have almonds in the orange trace.
We put these together, blueberries at about a 0.48 water activity, and their isotherm, which is a different shape you'll notice, then the almonds and the almonds came in around 0.3. Let's talk about the almonds first, and you'll notice that trace, that isotherm there is quite flat, which means that we're going to have a big difference in water activity and a small difference in moisture content. Circling back to that pecan example, if we just are able to measure moisture content and just really a wide range of moisture content, we're going to get a ton of change in water activity. That's not what we want. We want to know what we're getting.
For the blueberries, we're going to see for the same range of water activity, a bigger change in moisture content. We can take those relationships and we can blend them together. So when we do that, in this specific example, I have picked where we have mass, twice as much blueberries as almonds, and we've blended them together. You'll notice that there's two things on this graph that I haven't discussed yet. One is the green trace and that's the combined isotherm. That's the combined relationship of these two together. We can predict that. The other is a blue dashed line going up and down at around 0.45 water activity. That is where the water activity is going to end up once we put these things together.
We can know that, if we have the isotherms and we know where everything's starting, we can predict where they're going to end up. We don't have to actually do it yet until we know this is in a good range. When we look at the final water activity, it's at 0.45, the blueberries started at 0.48. You can definitely see here where the blueberries are the major contributor for where that water activity is going to end up. Now, the next step to do is to think, is that a good place for the blueberries? That's probably a fine place, it's already very close. Is that a good place for the almonds? That's a good question. Is this going to give us a soft almond, if we put these together at this ratio, or are these going to be at 0.45, is that going to be too soft for our almonds? This is something that we have a tool for.
ZC: Yeah, sure. This graphic that we've been looking at, it's derived from a graphic on the moisture analysis toolkit. This is a software that we worked a lot on just this last year updating and making it really user-friendly, but in that software, there's a mixing ingredient tool that you can do exactly what we're looking at now. You take an isotherm for each of the ingredients that you're adding. You have to think about which way the water's going to move. You may want an absorption curve for your almonds and a desorption curve for your blueberries, but you can use this tool to get the predicted final isotherm that you pointed out earlier in green, that isotherm can actually be used to predict shelf life and start to even make packaging decisions on a product or a mixture that you haven't even made yet.
You said this a little bit, but I just want to clarify that if you have these isotherms and you can sit at your desk and work through these mixtures, think about different ratios or think about the implications of your almonds changing to a different water activity before you even go and make that product. I work with a lot of teams that use this tool. Generally the feedback that I get is that they can release a product four or five times faster because they don't have to do all of these physical trials and wait and see if texture changes. They can start to look at these mixtures right there on their computer. They make an internal library of isotherms with all the different ingredients that they work with so that they can really speed up their R&D processes.
In the toolkit, it will show you the final water activity. It will show you the isotherm, and then again, it will give you the coefficients for that curve so that you can look at things like shelf life. These are some of the things that we've touched on in the past. We have a webinar specifically on shelf life, and another webinar specifically on isotherms and I'm sure we will have those links listed below. If those things sound helpful to you, definitely check them out. Also, we're always happy to demonstrate the software and walk it through with your team to show you what it may look like.
The business case
MG: This is a good place to talk about what can impact your profitability, and talk about some actual business cases, some things that we've encountered as we've worked with customers who work with these natural ingredients.
First I thought I could talk about one of the impacts that is a major issue and needs to be considered. We've talked about the product themselves and making sure we know what we're getting, but what happens after the fact, there are other things that we haven't discussed quite yet, which is storage conditions and temperature. The reason I want to bring those up is because generally, if you're increasing a temperature, you're increasing water activity in almost all ingredients.
Let's say you've produced a bar or a snack mix, and it's fine in your facility. Then it gets sent around the world in a truck, let's say that's hot, or a shipping container, the humidity and the temperature can really affect that product. Specifically, temperature can increase the water activity past a point where it's going to be safe and you can start getting mold growth where at your facility, it was fine. Now that relationship on how temperature affects it is also dependent on the product itself. It's something that you can study. You can actually find out the relationship of how temperature affects your product. But in general, I just know that increasing temperature is going to increase the water activity and to be able to account for that.
ZC: From my own experience, a lot of times when I'm consulting with other food scientists, one of the main concerns is avoiding a recall. From what I've seen, a lot of recalls will happen because the storage temperature or the shipping temperature gets a little too high. then the water activity reaches a threshold that we've touched on earlier in our different sections. But that water activity gets high enough for microorganisms to grow. That really shows the power of having an isotherm and understanding one activity. Because if you have this data and you have things under control, then there's no reason that you should ever have a recall and these things can cost millions of dollars. They can hurt a reputation. Having the right data and knowing how to use that data can help avoid any recall.
The last thing that I want to focus on is just a business case scenario. This is with a client that METER Group works with. I've shown a very similar example in the past for pet food, but I'm going to use the same format today to look at a prune producer.
We work with a prune producer who has a really big annual production of about 30,000 tons and their target further prunes was 30% moisture content. Even though we're talking about moisture content, we then turn to water activity and create the isotherm curve to understand how much we could increase the moisture content while keeping the quality and the safety that they want. By using the isotherm, we saw that they could increase by 0.5% moisture content and also reduce their variation and prevent any of their products from going above 0.7 water activity.
Their new target was now 30.5%. This allowed them to increase their production. Now they have a higher yield. Because they're selling this for a price per ton of $3,250, that means in one year, just that small change in moisture content resulted in an annual yield increase of almost half a million dollars. This is just one product. again, just a small change in moisture content, but by changing or by looking at water activity, by understanding the isotherm and then by implicating or introducing some control, some environmental controls, and looking at their packaging, it's really easy to make that adjustment and then to go to bed, knowing that your product is going to be safe and not having to worry about a recall happening.
This does a really good job of showing a business case. If there's a specific product that you work with or something that you'd like us to put a business case together for, it's really easy for us to do. We work with all types of products. We've just been focusing mainly on blueberries and almonds today because that's what we had available. It's really easy to demonstrate the isotherms and the moisture migration and these different things that we've touched on. But again, if there's a specific dried fruit or nut final product or ingredient that you work with, I'm sure that we've already worked with it in the past.
MG: I just wanted to mention to you, we did focus on blueberries and almonds this time just to keep it really simple, but this would expand to the theories that we're talking about, expand to everything, any food product. So if you are working with nut butter, the things we talked about are going to be similar, they're going to be impacted in the same way. If you're using a fruit paste, like a date paste as a base for your bar, it's the same story here. I just wanted to know that when we're talking about these things in general, it really is a generalization in that it applies to so many things.
Another thing too, we were focusing on microbial growth and yield and everything mostly, but it could be other things too. First, we were looking at isotherms in the way that water activity impacts different reaction rates. Let's say if you're having a bar or a product that has a specific nutritional additive to it, or a nutraceutical or a functional food, it's going to apply to those as well. All these series are going to apply to that too. You need to make sure that if you're making a claim like that, that you'll be able to maintain that claim.
ZC: Let's go ahead and turn this over now to the Q and A section. We'll take a couple questions and then again, if there are any extra questions you can reach out directly to us, or we'll have some contact information here at the end, so that we can make sure to answer all your questions.
Q&A Session
How quickly does moisture equilibrate between ingredients, and are there ways I can influence this?
MG: I'll take that one. It can take a little bit of time. It depends on that relationship of how quickly something takes up moisture. When we did our examples of the blueberries and the almonds, it took about a week to put them in a different water activity and then let them be. But if you're looking at most things, when they're packaged, they aren't expected to be consumed in a week. That is something that it will eventually, just to keep in mind, it'll eventually get there. It might be depending on the product, faster or slower, but it all will get to be equilibrated inside that package. You can be assured that will happen.
When you have a bigger difference in water activities, that is going to be the driving force. If you're able to get your water activities closer together, then you, number one, don't have as much moisture migration, and number two, it'll come to a good equilibration quicker. It's always good if you can try to minimize the difference. I definitely recommend that to try to get your activities to be similar, then you won't have as big of an issue of that moisture migration in your product.
How can I use moisture content measurements to predict moisture migration?
ZC: I can take this one. This goes back to one of our earlier sections that a lot of people think that they should be using moisture content because of its quantity. Maybe it makes a little bit more sense to them, but what I hope that we've shown in this webinar is that it's really the water activity that you need to keep in mind.
The water activity is a measure of the energy of that water, and it dictates which way the water wants to move. Water always wants to move from a high energy state to a low energy state. In the example that we looked at, by understanding that the water activity is higher in the blueberries, that water wants to move from the blueberry towards the almond to reach an equilibrium. Don't worry about moisture content, focus on water activity if you are thinking about moisture migration.
If my product gets packaged at a water activity level very close to microbial limits, how much variation in temperature after packaging would allow it to get moldy?
MG: You do want to stay away from that microbial limit. You don't really want to get too close. what I mean by that is if I had to say, and I really hesitate to say how close you can get, because it really depends on the product itself. What is its relationship with temperature? Meaning is it really impacted a lot by temperature and that, like I said, this is something you can find out and it is different for different products. But if I had to hedge it a bit, I would say 0.1, I would definitely keep you in the safe zone. We have the microbial limit of 0.7 water activity. If you're around 0.6 that's probably as close as I'd really want to get.
Remember in that water activity range, there's texture things happening too. It's a lot to consider, but if you're focusing on microbial limits, I would recommend something like that.
ZC: I see the same in a lot of industries and fruit and nuts and other products usually setting what activity of 0.1 below the microbial limit is a pretty safe bet. But I will also add for this question, if you were right at 0.7, let's say right at the microbial threshold at room temperature, as soon as your product leaves your facility, if it experiences any temperature higher than the temperature that you tested at, then as soon as you go up in temperature at all, you run the risk of what activity increasing. As soon as that happens, microorganisms can start to grow. There's a balance there between understanding the temperatures that your product may experience and then also setting what activity low enough that you're safe. If your temperature goes up, you're still going to be below 0.7 or even 0.6, five.
MG: I wanted to mention quickly if I could, two other things, these are natural products. Most of the time they don't have a kill step. I mean, if you're roasting a nut, then that should, if you're doing it right, I guess you could say. It would be killing any microbes, but for a lot of the stuff, things can still be on the surface of your natural food, and they won't grow if you can keep your water activity low, but if you do get high and even higher, we're talking about molds that's pretty low, 0.7, but if you get higher, you can run the risk if it's in a different environment or your packaging isn't good enough to throw your humidity high, which is a factor now, or you can have E coli or salmonella start to develop. That is much higher water activity, but just remember that those things can still survive in natural foods, they just can't thrive or grow if you can keep the water activity low.
The other thing I wanted to mention too, is the impact of packaging, which I know you have a lot of experience with. If you want to discuss something concerning packaging in relation to temperature and such.
ZC: Yeah, sure. Just really briefly if you're producing a product that is close to a microbial limit, one way to keep your product within your ideal water activity range is to consider your packaging, the lower your water vapor transmission rate for that packaging, the better you'll be able to keep it within the range that you need it to be, even at different humidity or different temperatures. Again, we have another webinar that goes into this in much more detail, and we'd be happy to discuss packaging with you.
Is it necessary to check moisture or water activity at any stage beyond initial ingredient mixing?
MG: Yes.
ZC: Yes, definitely.
MG: Yes. Recommend of course water activity as we've discussed, because moisture content isn't giving you the full picture to be able to avoid some of the problems that we've talked about. Then the process control. You can achieve that a lot better with water activity and it's good to test the incoming ingredients, but post is also very valuable because then you'll know what you've produced. Is it at the level that you are expecting? Do you have any more comments?
ZC: Yeah. I'll just say that successful companies that I see who really have control of the water in their food or the ingredients. Do measure at the start, they will only accept ingredients if they're within a 10% range or they set a specific range because that variation kept in mind will be carried into the final product. I recommend measuring at the start your ingredients during production. We even have an inline solution now so that you can always hit your target, what activity, but then still again, at the end, you should be measuring your final product right when you put it into packaging. Then we'll even see companies who do like a shelf life study, who will measure water activity over time. it's definitely something you want to monitor through the entire process. By doing that, you can avoid recalls or any of these other issues that we've talked about today.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
