Webinars
Substituting sugar: Pros and cons of 5 top alternative sweeteners
Demand for healthier snacks and treats continues to grow. Innovative new sweeteners abound. But in the scramble to develop the next great clean-label snack, food companies are finding that each sugar substitute comes with a special set of challenges.
Since substitutes don’t perfectly mimic sugar’s characteristics, formulators are left with a complicated job: Finding new ways to achieve the sugary taste, texture, shelf life, and appearance that will satisfy consumers.
Join Mary Galloway, head of the METER Food R&D Lab, and Dr. Zachary Cartwright, lead food scientist, on November 15 as they present original research that addresses the challenges that come with using sugar alternatives. They’ll cover:
- The pros, cons, and frequent challenges associated with 5 top alternative sweeteners
- The scientific concepts that explain sugar’s unique characteristics
- How formulators can use water activity measurements to minimize the challenges that come with sugar substitutes
- How blending different sugar alternatives can yield better results
About the presenters
Mary Galloway is head of the METER Food Research & Development Lab. She specializes in using and testing instruments that measure water activity and its influence on physical properties. She has worked with dozens of the world’s largest and most successful food brands to solve moisture-related product issues.
Dr. Zachary Cartwright is lead food scientist at METER Group. He holds a PhD in food science from Washington State University and a bachelor’s degree in biochemistry from New Mexico State University. He is an expert in isotherm analysis and the use of the Vapor Sorption Analyzer (VSA).
Transcript, edited for clarity
Dr. Zachary Cartwright: If you're at this webinar, you probably already know that alternative sweeteners are a big deal. There's been a lot of consumer pressure and governmental pressure for sugar to be removed or reduced in a lot of different food formulations, and hopefully what we have to share today will help you understand that better. Now, this is a huge market. It's valued somewhere over $18 billion, and according to the research we are able to find, this is going to just grow over the next decade, and so it's something definitely to pay attention to, whether you're concerned about the financial impact or you're a food scientist, and you want to understand which alternative sweetener you should use if you're replacing sugar. Mary, what are we going to go over today in this webinar?
Mary Galloway: Yeah. We're going to talk about specifically what sugar is and how it relates to moisture and the interaction that those create. Those will also affect the quality characteristics, and we're also going to discuss what it means to be Clean Label, because not only does a consumer want to have reduced sugar, they want some other things to go with it as well. After we've talked about that, we're going to talk specifically about five alternative sweeteners that we've been studying and give you our data and our information that we've gained about those.
THE REAL THING: HOW SUGAR WORKS
ZC: All right, let's start off by building a foundation. We're going to talk about what sugar is, some of the quality perspectives that you have to keep in mind when having it in a formulation, and then we'll also talk about how sugar relates to moisture in food formulations. Generally, table sugar is sucrose. This is a disaccharide of glucose and fructose. It usually comes from either sugar cane or from sugar beets, and usually, it's produced in ... The top producers include India, Brazil, and Thailand, just to name a few places, so Mary's going to tell us a little bit about sucrose and what to consider when using this in a formulation.
MG: Number one is the sweetness. It is the standard that everything is compared against. It also gives enhanced flavors, so besides just the sweet, as it chemically breaks down, it's going to bring out other flavors in your product. It also is influencing leavening in three different ways actually, so we have aeration caused by sugar trapping air in the fat molecule, we have yeast, the sugar is used up by the yeast, and then it also stabilize egg whites while it binds to proteins, and lastly, it will deepen the color, so it uses the Maillard reaction to react with amino acids, and you'll get that nice, beautiful brown in your baked goods.
ZC: The way that moisture starts to fit in here is that sucrose is a polar molecule, so there is going to be a hydrogen bonding effect with water. You're going to have positive areas of water molecules interacting with the negative areas of sucrose, and because of these interactions, sucrose is a really good humectant. It's something that will bind or lock up water so that it's not available for other reactions or microbial growth, or other things you might be concerned about in your food. How else does sucrose interact with water?
MG: Yeah, it's actually a major component of formulations, is that reaction together. How does water and sucrose bind together? Number one, it creates tenderness because it'll absorb the water, reduces gluten formation, so you'll get, reduces the staling of bread, and it also delays the coagulation of proteins as well. It adds a crunch, so as you bake something, the moisture's going to evaporate on the outside, and the sugar is going to recrystallize. As we already mentioned, it's a really great humectant, which means it just straight up binds with water and increases the moisture content, so if you want something to be more tender or have more moisture in it, then adding sugar is really going to help with that.
It also is really good at extending shelf life. Because it does lower that water activity, it inhibits the microbial growth, but it also starts limiting chemical reactions and just improves overall stability. Lastly, it lowers freezing points, so if you're looking at ice cream, what happens is it will bind with the water, and then it'll keep that water from crystallizing in those frozen dairy products, so those are all really important and huge effect in all products that need to have sugar in them.
SCIENCE & PRINCIPLES: CONSIDERATIONS WHEN SUBSTITUTING SUGAR
ZC: All right. That's a great point, Mary, and that's why in this next section, we're going to talk about the science behind substituting some of the sugar with alternative sweeteners. There's a lot to keep in mind, like you just went over all of these different characteristics are impacted by sugar. It's not just sweetness that we're looking at, so we're going to start to talk about some of the characteristics, so final products to keep in mind, and then also try to define what it means to have a Clean Label and how these sugar substitutes fit into that. As far as replacing sugar, sugar is really complex and much more sophisticated than it might appear at first.
As you change it, keep in mind that this has a really big impact on the water activity. This goes back to when we mentioned humectancy. Anytime you change water activity, this can have an impact on the physical characteristics of that product. You have to keep in mind the texture that you're after, if you're going for a certain crunch or crispness. Whatever that texture is, as soon as you remove sugar, the texture may change as well. You have to keep in mind chemical effects, so as you change the water activity, certain chemical reactions may increase or decrease in speed.
Also, if you replace with a sugar that's not going to brown the same, then there's going to be an impact on browning reactions, and then there also may be a biological impact, so as that water activity changes, this may increase or decrease the susceptibility of that product to a biological growth of certain microorganisms. Now, consumers are looking for lower sugar in their diets, but they also want a natural or a Clean Label. Mary, can you tell us a little bit about what it means to have a Clean Label and how this fits our topic?
MG: Yeah. I found this really interesting quote by Melanie Goulson, saying, "Replacing the functionality of sucrose is difficult enough, but food formulators must also consider regulatory issues, taste profiles, nutritional targets, digestive tolerance, shelf life issues, and product claims," so that's a lot to take into account when you're a formulator, trying to replace something that seems so simple as just switching out sugar. Clean Label is a movement by consumers, and it's really about perception, and that is where it gets a little hard because it's not well-defined, and it's not regulated either. When a consumer is looking for a Clean Label, what they're looking for are what they consider natural ingredients, so no artificial flavors, colors, preservatives, or synthetic additives. They're also looking for simplicity, so they want to have things that are more recognizable in the name, things that sound less chemical or artificial. They want transparency.
They want to have information on how those ingredients are sourced or how there have been manufactured or made, and they want minimal processing, so they want things that are simple, to be understood in how they got them in their product, but that can be very confusing in a way because, as an example, when you are trying to make, let's say a nut milk, that takes a ton of water and a ton of processing, and is not a natural process, but it's considered pretty Clean Label and natural because it's a plant-based product, so trying to navigate all of these perceptions that a consumer has in formulation can be very difficult. I do want to mention one more thing, which is taking all this into account, consumers are picky, and they don't want anything to change, so if they've had a product that they've enjoyed for many years, they don't want those attributes of that product to change, so it's also, makes it very difficult because when you start taking out things to replace them, you're going to introduce other flavors and textures in other properties that you have to try to navigate as a formulator that are not going to be desirable, so even if you are successful in producing this Clean Label product, if it doesn't taste good or doesn't have the attributes a consumer wants, they will not buy it, so that's tricky.
SPECIFIC SWEETENERS: BALANCING PROS & CONS
ZC: Yeah, and that's a great point. Anytime you change your formulation and you're trying to keep the same exact taste profile, it can be really difficult to do, but in the next section, we will start to talk about some of these sweeteners and their pros and cons as it relates to formulation. As we dive into these specific alternative sweeteners, we're going to talk a little bit about frequent challenges with them, in addition to those pros and cons, and then we will show some data on how they interact with moisture. Let's dive into the first one, or maybe let's list them for now, so we're going to look at stevia, sorbitol, erythritol, maltitol, fructose, allulose, and then we will also have some honorable mentions.
MG: Yeah, that was a hard list to cut down so-
ZC: Yeah, yeah. I mean, there are lots of options out there, so cutting it down to five was hard. If there are questions out there about some of the ones that we don't go over, we're happy to take those. Let's start with stevia. Mary, why don't you tell me some pros and cons about stevia?
MG: Yeah, I would say probably, one of the biggest pros of stevia, it's readily available, and people understand and accept that as a naturally good sweetener. It is generally ... Because of the size of the molecule of stevia, it really has a varied amount of sweetness, so it could be 50 to 300 times sweeter than sucrose, so it's a big range, which could be a plus or a minus, depending on how you look at that. It's also pH and heat stable, which is really good if you need that in your product. One of the downsides is that it doesn't brown, so if you're looking for that in a baked good, that's something to consider, and it does have a bitter aftertaste, and honestly, the range of the sweetener could be a negative as well because you have to make sure that what you're sourcing the first time in new formulation has the same kind of sweetness in subsequent formulations so that you get the same source and sweetness level as you're expecting in your product.
ZC: The only other con that I might add, and this is true for most of these alternative sweeteners, is that a lot of them can have a negative impact on people's guts. They can have gastrointestinal impacts, so that's definitely something to keep in mind for all of these sweeteners. Let's move on to the next one. The next one is sorbitol. This one, from my understanding, is a good humectant, so that would be a pro. It's also a nutritive sweetener, so this one is going to have some caloric impact compared to some of the other options. What other pros have you seen for clients or companies that use this sweetener?
MG: Right. It is a sugar alcohol, and this one, in particular, is less sweet. It has about 60% sweetness compared to sugar. It's also a good plasticizer in baking, so it slows that staling process, so it would be a good replacement in baked goods if you're looking for something that's going to slow that staling down, so it does a good job in that, and not a lot of the other sweeteners have that as well. It is a nutritive sweetener, which means that it's not zero calorie, but it does as a sugar alcohol, and most of the sugar alcohols actually fall into this category, where they are having some kind of GI distress, so that is a pretty common consequence, I guess you could say, of these alternative sweeteners, and that is the case for sorbitol as well.
ZC: Yeah. From my understanding, this actually has been used in a lot of laxatives in those types of formulations, so definitely be careful with how much of this you may be adding to a formulation. The next one that we would like to talk about is erythritol. This is another sugar alcohol. What are some of the pros and cons here, Mary?
MG: Yeah, for erythritol, it's similarly sweet to sorbitol, so it's like 60 to 70% sweet as sugar. It has a minimal effect on blood sugar level, which is really great, and it's also non-caloric, so it has hardly any calories to it, and it's pretty readily known. Most people know what erythritol is. One of the things that we found that was really interesting about erythritol is it has low solubility in water, and that is a major factor, so even though it kind of has these great things in it, it is something to consider when you're using formulation, that it's not necessarily going to stay in the form that you need it to be. It also has a slightly cooling effect, and so that makes it more limited in its application, but using it in like ice cream or perhaps a gum has a good response with that.
ZC: All right, and you mentioned that low ability, and we do have some data on this specifically, so why don't you tell us a little bit about this chart that we're looking at, and how you're able to see the loss of solubility, specifically in this type of data?
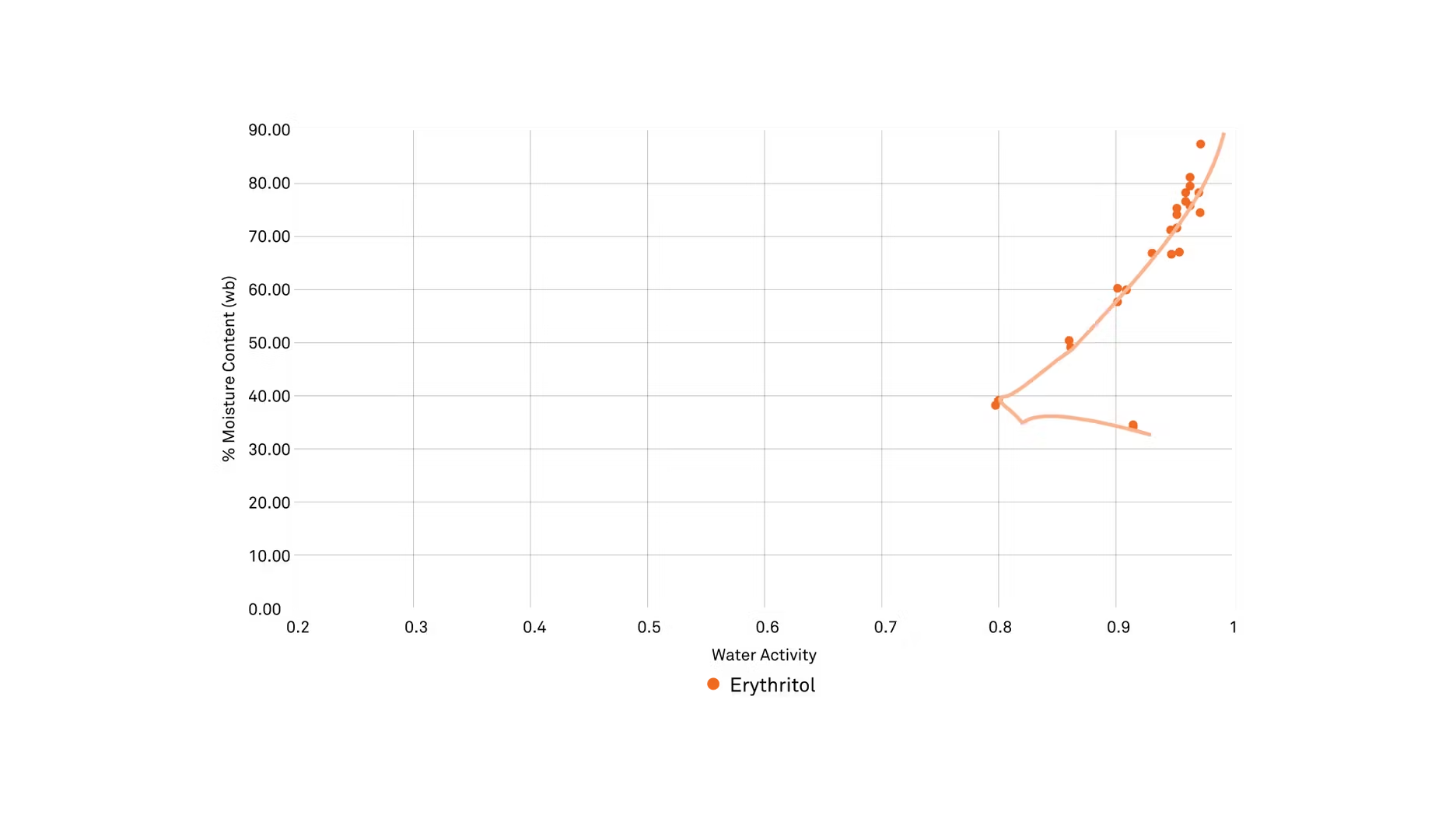
MG: Yeah, absolutely. When we first were running a study, we were setting all of these natural sweeteners and alternative sweeteners, and one of the things we were doing was making solutions out of the sweetener in water at 25 degrees C, and then seeing how well they reduce the water activity, so basically, we're just checking that humectancy, how well do they bind with water and reduce that water activity like sucrose could, and we couldn't do it with erythritol, which was really interesting. It just stayed in a crystalline form. What we ended up doing was making a solution and heating it. We actually boiled it, and then what we did is we boiled it down and periodically took a sample and got a moisture content and water activity, and we manually built an isotherm, a desorption isotherm of erythritol. It worked really well actually, until we got to about 0.8 water activity, and at that point, you could physically see crystallization happening in there, even at that high heat.
What we found is really interesting is that after that, we did get one more data point after it started to crystallize. If you look at the chart, you can see where it kind of takes a zag, and it goes from 0.8 ... It goes up to about 0.9, even though the moisture content is reduced, so it has this funny little jag, and what you're just seeing there is the actual crystallization of the erythritol, and what happens when it crystallizes, that structure becomes rigid and it kicks out the water that it was bound to, so you see that it's going to drop in moisture content, but it's going to increase in water activity. Now, what will happen if this is in your product and it does crystallize, you will have crystals of erythritol in there, and you're also going to have added water. You're going to actually have liquid water come out into your formulation, and those are undesirable.
ZC: Yeah. I mean, if that's happening, then this product suddenly becomes susceptible to maybe microbial growth, plus you have that bad crystal effect, so definitely something to keep in mind for this specific alternative sweetener. Let's move into a couple more. The next one is maltitol. We decided just recently to add this to our presentation, or to our webinar, but why did you decide to add this, Mary?
MG: It is in many things. I started doing a quick, little search on regular formulations of candy, and cakes, and various things like that, and just to see what companies are changing, what are they putting in there to replace whatever their sweetener is. Sometimes that sweetener is sucrose, sometimes it's high fructose corn syrup. It's various things, but half the time, maltitol came up, so we wanted to put something in here about it. It's not readily available for consumers, but it is for formulators, so it's a sugar alcohol.
It's nearly as sweet as sugar, so the range I found was like 75 to 90%. It had the most similar characteristics to sugar than as other alternative sweeteners, so we're talking about sweetness and bulk. It dissolves similarly as well. One of the things that was a high point, and probably why I saw it a lot in candies and confections is that it has a high melt point and it has kind of a creamy texture, so it makes it stay in that nice, amorphous state in a candy, and also gives kind of creaminess to chocolate as well. The taste was really good as well, so it didn't really have a metallic aftertaste, or licorice, or acidic, or any of that other stuff that you do tend to get with sugar alcohols.
It is what we call a nutritive sweetener, which means it does have some calories to it. The other thing I found was really interesting too, is that its glycemic index is really similar to sugar, so meaning, it will kick your blood sugar up, so it's good for formulations, does a lot of good stuff, but as a consumer, that would be important to know that it is going to definitely affect your blood sugar.
ZC: All right, let's move on to fructose. This is a fruit sugar, naturally occurring in things like apples, as well as grapes and pears, so fructose, what are some of the pros and cons with this one?
MG: Yeah. It's a simple, natural sugar, and it's also half of what makes up sucrose, so it's a good humectant. It's good at lowering that freezing temperature. It browns, so it has that nice Maillard reaction, and it will ferment, so all these kinds of things that make sense with the fruit sugar. However, if I say high fructose corn syrup, nobody's going to like that, so there is a kind of a connotation about fructose, and especially in that form that isn't really good.
It is a nutritive sweetener. Like I said, it's half the sucrose, so it has the same number of calories that sucrose does, so four calories per gram, and eating too much fructose can have adverse effects on your body so ...
ZC: Yeah, I was actually surprised when we were doing research for this webinar. There are some diseases or disorders that you can develop from too much fructose. I mean, glucose is the body's preferred source, so fructose ends up going to the liver, and so that it can be converted, but just something to keep in mind with fructose, so really, any of these, really limiting the amount or thinking about how much is there and maybe some of the health concerns, as well as some of the flavor or food quality concerns as well. Our last couple here are allulose and tagatose. Aren't these fructose, or how are they similar to fructose?
MG: Yeah. These are new on the market and very hot right now. Allulose especially, we found this a lot in gummies and all sorts of things. Allulose and tagatose are what we call an epimer of fructose, which means it has the same chemical makeup, but it's arranged slightly differently, so that does change some of the properties that they have. Similar to fructose, they're both good humectants, they lower the freezing temperature, and they have a nice browning effect, so those are all definite pluses.
For allulose, it's about 70% as sweet as sucrose. It can have a slightly bitter taste, but it's very popular like keto-friendly sweets. Tagatose, even though it is the same structure as fructose and allulose, it still has slightly different properties, so it's a little sweeter. It's about 90% as sweet as allulose, but there are some calories involved with tagatose that you don't see in allulose. There is no bitter taste.
Currently, the FDA has declared it and added sugar, and they're currently fighting that. I imagine that tagatose, you're going to see that a lot that more in the marketplace being available. I can think of actually one specific company that has found a new way to produce it. One of the reasons it's been difficult is because it's expensive to produce tagatose, and there's been a new development on how to produce it, so I imagine that's going to be coming in the future so that you will see tagatose more readily available.
ZC: You're right, it is a rare sugar, and this is why we see companies like Hershey's, who has invested in Bonumose, and I believe this is what they're producing because they're using their own enzymes and trying to make this rare sugar more affordable so that this can be in some of the sugar substitutes and some of the formulations. We will look at all of this in some of the data that we've collected. Let's look at some of the data that we've collected to compare the humectancy between sucrose and some of these alternative sweeteners. How did you set up this experiment, Mary, and tell us a little bit about this table we're going to look at and how to read it?
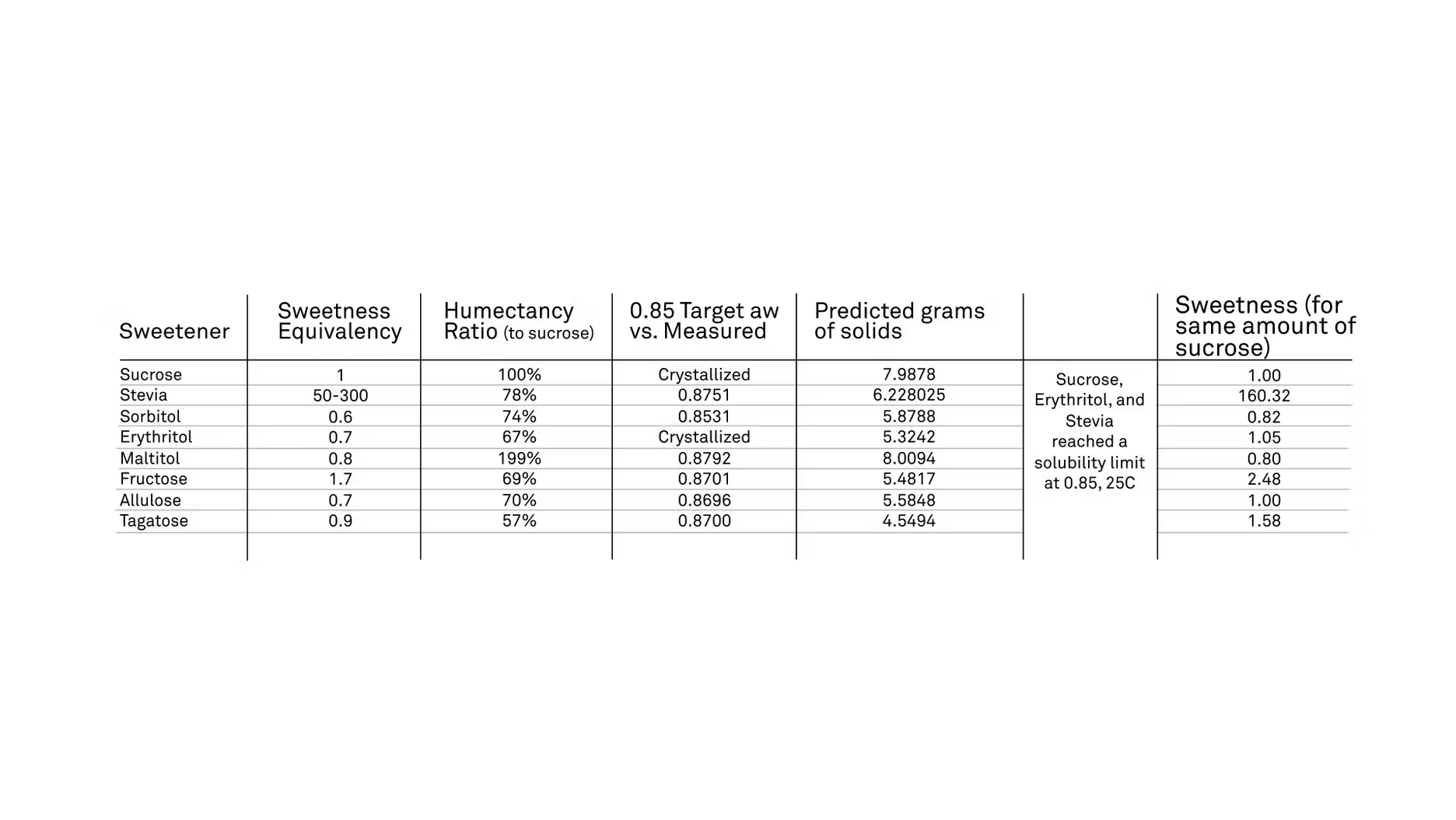
MG: Right. We wanted to know, since sugar is a good humectant, how do these other sweeteners compare? Granted, this is just one aspect of what sugar brings to the table, so it has a lot of other attributes that are needed in these formulations, but we're going to focus here on humectancy. Basically, when I was talking before about these manual isotherms, we did that for all of our natural sweeteners here, and we were able to produce a relationship between water activity and the moisture content, and relate that to, in terms of sugar, how well does it compare to sucrose? We have a sweetness equivalency, so I wanted to kind of put that there, varying amounts.
Most of these sugars are ... Well, we're kind of 50/50, really. Half of them are a little less, half of them a little more. Based on the relationship that we found in the lab, we were able to kind of calculate how much of mass amount of these sweeteners would we need to replace a similar amount of sucrose, so if we have sucrose and we targeted a 0.85 water activity, meaning, we made the solution, that would give us that specific water activity, so that's what we're talking about humectancy. How would that compare to sucrose, and so I have this humectancy ratio.
100% would be the same amount as sucrose, and then all the other ones, and most of them are less, it would take less of that sweetener to produce a similar effect, a similar binding effect to the water. That was a little bit of a surprise because sucrose is known to be a really, really good humectant, and then we turn around and we look at these natural sweeteners, and we say, "Ah, they can actually be better at it than sucrose can." I found that was really, really interesting. One of the things I wanted to add on the table too is, "Okay, so what if we added the same amount of sugar in our formulations? What are we going to get sweetness-wise?," and so I have this little bit on the end of my table, sweetness for same grams of sucrose, so if we added the same amount of these sweeteners, how sweet are they going to be, and so I just took an average for stevia, so like 160 times as sweet, and I felt maybe gave another way to look at this data.
If we use the same, if we just gram for gram replace sugar, what are we going to get humectancy-wise, and also, what are we going to get sweetness-wise, so you can get a similar humectant effect, but you're also going to get a lot sweeter, so all part of that formulation consideration, honestly.
ZC: Then, we also collected some isotherms on these different alternative sweeteners. This is kind of our bread and butter to look at the relationship between water activity and moisture content, so let's first look at the sugar alcohols. That includes sucrose, or we're going to look at sucrose compared to sorbitol and erythritol. What do we see from the isotherm that you might not see if you didn't have this data?
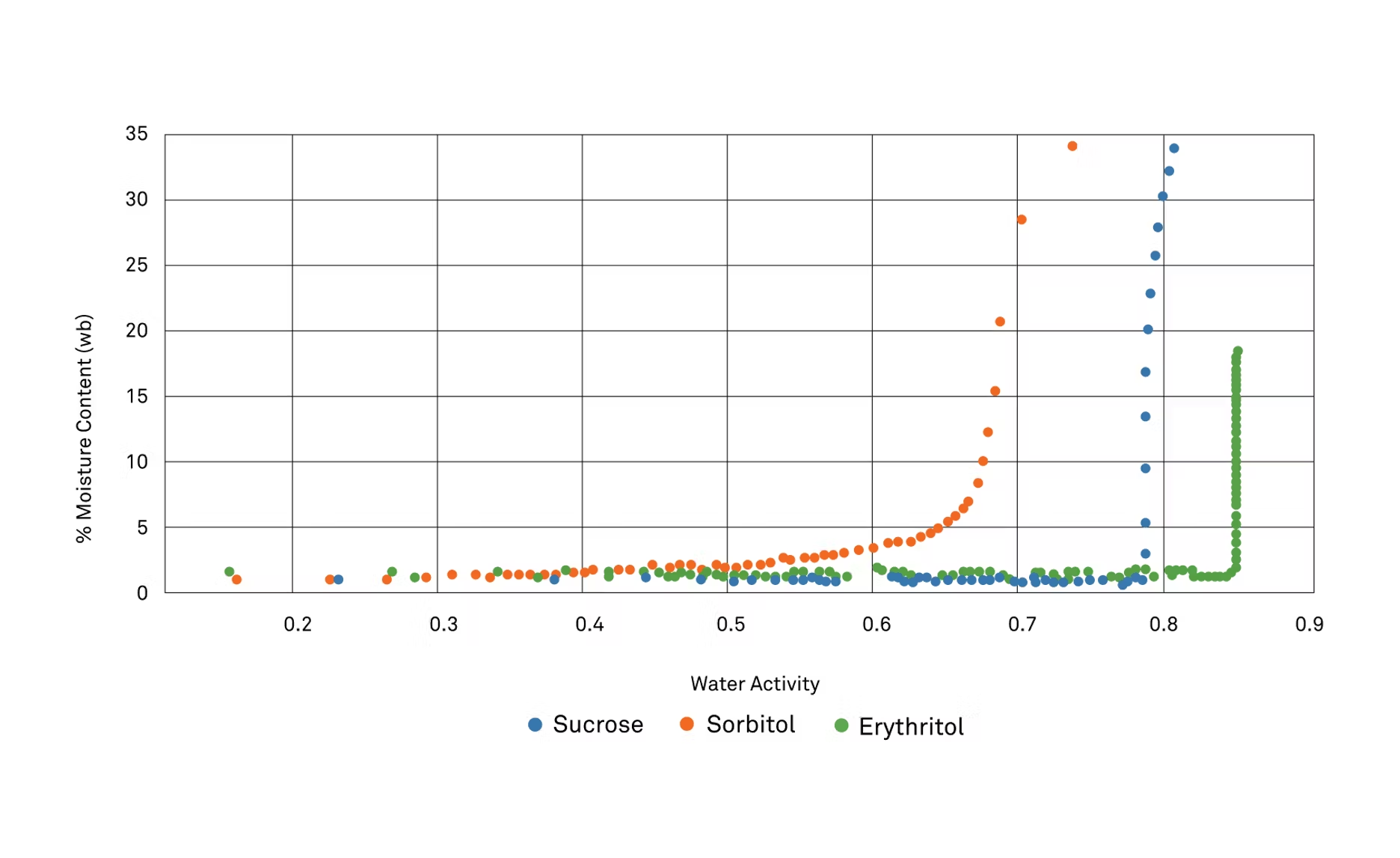
MG: Right. One thing that was really apparent is that the erythritol is so flat. It's very similar where above, let's see, about nine, two water activity, it just goes straight up. What is happening is this deliquescing, but that's also its ability limit, so when I was talking before about that crystallization problem that we are finding with erythritol, that's what we would see at 25 C. We had to really crank up that heat to get it to go into solution.
As I read some things online, that is a problem. A definite problem, so as we look at this isotherm, we'd be able to see that right away, that it's not going to take up moisture until it gets high enough. It'll finally dissolve a 25 C. Conversely, when we look at sorbitol, it does have a more gentle effect, and you can see that it does and did have a good humectant response, so it starts taking up moisture pretty much from the get-go. Around 0.7, it really starts to take off and bind with the water a lot.
That's some easy things we can just learn from looking at this isotherm. We can also see too, and as I alluded to before, when we did our solutions the other way, our manual isotherms where we were heating up and cooling it down and taking these samples as we're driving off the moisture at this high heat, they correlate, so where that point happens, where they start to crystallize, you can see in this isotherm too.
ZC: I maybe should have mentioned that these are absorption isotherms, so we're looking at the way that water is binding to these alternative sweeteners, and then we can really clearly see inflection points or deliquescence points where these are going into solution. Now, let's look at fructose and how it relates to a few others, so we'll be looking at fructose, allulose, as well as tagatose.
MG: Right.
ZC: What are we seeing here compared to the other graph?
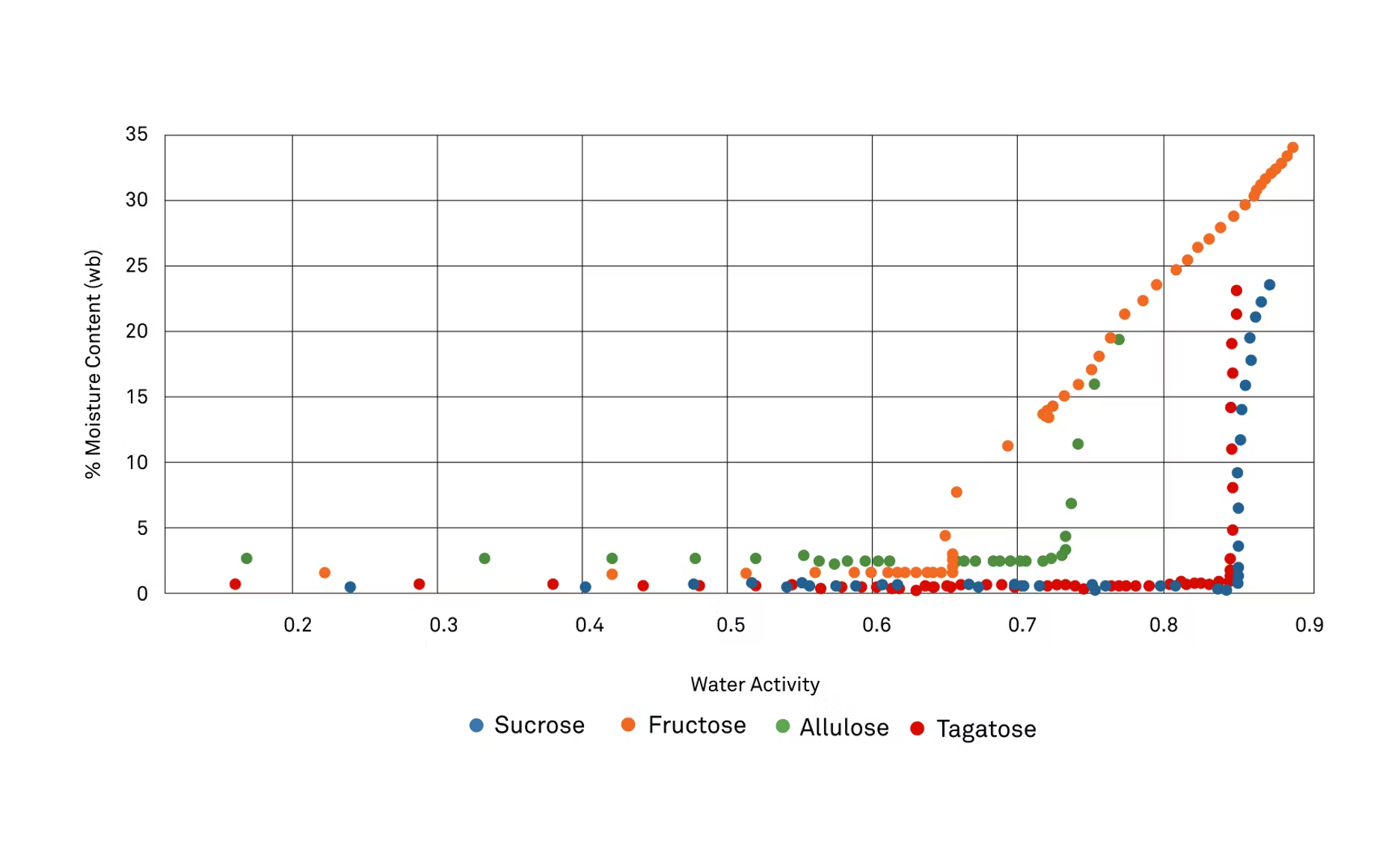
MG: Yeah, so this is quite interesting too. You'll notice that if we look at sucrose, which is the blue trace, and tagatose, which is the red trace, they're nearly right on top of each other, so they're very similar in their characteristics between the two. We kind of saw that as well when we were researching it, some of the attributes that tagatose has matched closely to sucrose. As we're looking here, you'll notice that fructose actually has the lowest water activity where it starts to dissolve, so that's nice, so it'll stay in that nice amorphous state. Then, allulose is kind of in the middle.
It's around 0.73 would be what I would say, and then we start seeing its response where it starts to take on moisture. I just wanted, before we kind of leave this topic, just really hit again, the importance of having your sweetener in an amorphous state because that way, it can bind with water. Once it's crystalline, the only way that it'll react with water is on the surface, and so you won't get these water-binding capabilities that you would see and desire in something when you're looking for the sweetener, so you must keep your sweetener in an amorphous state.
ZC: Then, let's finish off this section with a few honorable mentions. Like we mentioned earlier, we can't really go over every alternative sweetener that's out there. We tried to really hone in on the top five or so, but what are some of the other alternative sweeteners that you see either from clients that we work with or when we did our research? What did you come across?
MG: Yeah. Monk fruit extract was big. Very, very potent sweetener, so that's nice because you can add just a little bit of it to kind of boost that sweetness profile. It doesn't really add any bulk because it is so sweet, so it's just really for that part of it. Licorice root extract, we found that as well, but as you would imagine, it does have a licorice flavor, so that tends to be more limiting in that you would use it maybe in a strongly flavored candy or something like that.
Another one we ran across a lot was maltitol. It's also a sugar alcohol, about half as sweet as sucrose and about half the calories, so it still has that. It does resist crystallization, which is the major plus for isomalt, and they use it a lot in candies for that very reason.
OBSTACLES AND SOLUTIONS: BLENDING SWEETENERS
ZC: Again, if there are other sweeteners that we didn't include that you'd like to hear about, please let us know. All right, in this final section, we're going to talk about mixing some of these alternative sweeteners. We didn't collect data in our lab, but we thought it would be helpful to talk a little bit about some stories or anecdotal evidence about how different companies, making different products are able to achieve their goal and have the same texture and taste and so on, using a mixture of some of the sugars that we mentioned. The way that we're going to set this up is I will say the obstacle, and then Mary is going to give the solution. The first obstacle is related to high-intensity sweeteners.
Here, the obstacle is that you can only use a very small amount because if you have too much, it's going to really affect the taste or maybe have a negative impact on the gut. When using a high-intensity sweetener, what's the solution here?
MG: Yeah. It's going to be good for that nice, quick, sweet profile like the monk fruit we were just talking about, but we need to make up the mass different, so adding a bulking agent, maltodextrin, polydextrose, inulin fibers to replace the mass that's lost there.
ZC: The next example, and you mentioned this a little before, ice cream. If you are changing and using an alternative sweetener, then you're going to lose some of that water-binding, and there may be an effect on the freezing point depression. Well, what's the solution here for this type of product?
MG: Yeah. There is not just one, I want to point out, but as an example, this is one. You can use erythritol, as we mentioned before, because it does do a good job of that freezing point depression, which you need to be able to make an ice cream, but maybe you would add something else like stevia or some other bulking agents to fill in some of that to have a good mouth-fill on your ice cream, so it's still kind of a blend. You could start with erythritol for the freezing point depression, add a little stevia for the sweet, and then have to add a bulking agent to fill out and have the good creamy mouth-fill that you're looking for.
ZC: I'm getting hungry as we go through this. Next, let's do nutrition bars. I see this more and more. A lot of nutrition bars want to lower the calorie in that bar and make it as healthy as possible. The obstacle here is that when you remove the sugar, sugar actually has a huge impact on the texture profile of these types of products, so you might get a hardening effect that you were trying to avoid, or I see this all the time where companies replace a component like sugar, and then all of a sudden, their whole product is different. What could we do here as a solution if you're removing sugar for this type of product?
MG: Right. Maybe a good solution for this one is the allulose, and for the sweet, and then it does have a lot of good properties that are similar to sugar, that we spoke about before. Then, you can also add some soluble corn fiber, and that is also going to help bulk out, keep the good texture, maintain the taste and the shelf life. If you remember, one of the things that sugar does is it slows that staling process and that protein coagulation, so yeah, no surprise, and you look into it why that's hardening so-
ZC: We actually have a project right now where we're looking at some nutrition bars, so hopefully more in that later. Now, let's talk a little bit about taste profiles. You might have an obstacle where you have delayed sweetness, so with sucrose, you expect it to have a very specific taste. It's going to hit your tongue in a certain way, but as you start to use some of these alternative sweeteners, that sweetness might show up later, or it might not be the same, so how could we think about formulating for this?
MG: Right. As an example, stevia kind of has a delay in the sweetness and maybe a little bit of an odd taste after as well, so you could blend that with allulose or tagatose, which has that initial burst of sweetness that a consumer would look for. I mean, there's also going to be some other added benefits for that as well, but for that, if you're looking to get that quick burst of sweet, those would be good.
ZC: Then, what if the obstacle is an off-flavor? I've heard of monk fruits having kind of a melon rind flavor or off-taste, and stevia, like we've mentioned, might have a kind of a licorice taste, so what can you do to combat these off-flavors?
MG: Yeah. Interestingly enough, I've seen where they've actually combined those two together, and it actually neutralizes their off-flavors and provides a neutral sweet, so it's kind of interesting that they can have this kind of synergistic effect when you blend them together.
ZC: Then finally, what about erythritol? We've mentioned this a few times. It has kind of a bright effect or a cooling effect in your mouth. What might you do if you're using this specific alternative sweetener?
MG: Yeah, and stevia, I've seen that too, where they put a little bit of stevia in, and it kind of settles down that bright, that cooling effect. One of the things we didn't mention, and we were just talking about here earlier, was xylitol, which is very similar to erythritol, in that it has a cooling effect. You'll find it in gum, and it's also one of the reasons if you ever get like a cinnamon gum and it's always like a cinnamon mint, it's because of the xylitol, because of the cooling effect in that artificial sweetener.
ZC: Interesting. Then, this last example we have here is just a baked cookie, so if you're going to reduce the sugar and use an alternative sweetener, then there's an impact on the spread if you're trying to cook this, so how might you overcome this type of obstacle?
MG: Yeah. I wanted to put this example and discuss it a little bit because we've been talking solely about these alternative sweeteners, but there are other aspects we need to consider, and there might be other ways to adjust your formulation as well, that isn't just about sweet. Then, this is a good example of that, where you don't get the spread that you're expecting when you have a reduced sugar, plus an alternative sweetener, so one of the ways to do it is to actually increase your fat to flour ratio, and you'll definitely get that spread, and then you can reduce your baking time. That might be another way to look at adjusting your formulation to account for the lower sugar.
ZC: Just as a summary, I know we've talked a lot about sweetness, but you also have to consider those adverse flavors or some consequences, like the gut consequences.
MG: Yeah, consequences.
ZC: And you may need to add other functional ingredients. If you're removing your sugar, you may need to add an emulsifier or some type of stabilizer, or bulking agent, and like you said before, keeping it in an amorphous state is extremely important so that we don't get that crystallization in that water that's kicked out.
MG: Right.
ZC: Just as a quick recap today in this webinar, we talked about why alternative sweeteners are a big deal. We defined sugar and talked about how it interacts with moisture. Then, we talked a little bit about the science behind sugar and these alternative sweeteners. We looked at specific alternative sweeteners. We went into some pros and some cons.
We showed some data, and then had some honorable mentions. To wrap things up, we looked at some mixing or some anecdotal evidence of how some companies, depending on product type, are taking this challenge and figuring out a way of the right type of mixture to use for their specific case. That was everything that we wanted to go over today. Please make sure to subscribe to our YouTube channel, and also check out our podcasts. Now, we'll move into the Q&A section and take your questions.
Q&A SESSION
Can you discuss bulking ingredients, and what are the bulking effects of these alternatives?
ZC: Yeah, so we brought this up a little earlier when we were showing the obstacles and the solutions, specifically for high-intensity sweeteners, but Mary, what are these bulking agents?
MG: Yeah. What makes them a bulking agent and why you would need them when you have a different sweetener is because sugar is going to add a certain kind of mass, certain amount of mass in your formulation, and when you remove that, you need to replace that mass, so we have bulking agents that can be added like maltodextrin. Then, one common example of that is if you go to the grocery store and you want to have something like a stevia blend. Stevia is quite potent, so you would have a little bit of stevia and a lot of maltodextrin, so you see a lot of these kinds of blends. Maltodextrin, as I just said, is one of them, but it could be like polydextrose. Inulin is another one that's popular, or fiber. We talked about soluble corn fiber being a bulking agent because they're able to absorb water, hold onto it. They replace the mass, but they also can replace some of that water-binding that's lost when you're replacing sugar.
ZC: I'll just include that if you're a consumer and watching this, definitely check out the back of a label, and if you see that there's an alternative sweetener, you're going to also see some of these bulking agents listed on that label.
What natural alternative sweetener mix best imitates sucrose?
ZC: That's a tough question because depending on your goals, it might be a different mixture or several of these alternative sweeteners, so we'd really have to know a little bit more about the exact product and the goals, but as we looked before, some of these alternative sweeteners do behave a lot like sucrose. For example, when we showed the isotherms, tagatose was extremely similar to sucrose, so if you're looking for the same type of absorptive properties, that might be a good substitute or something to include in your mix. What else would you include, Mary?
MG: Yeah, that's a good consideration. It's hard to get a specific answer for what is the best. If you're looking at sweeteners, maltitol was really good, allulose was good, tagatose was good for looking at the sweetness equivalency. I also had some good humectant properties as well, but then, when you start looking at other ones, sugar alcohols tend to, not brown, let's say, but maybe you have a baked item, so that would not be maybe good to use, so allulose or tagatose may be better in that regard, but then, maybe you have a different formulation where you're looking to have low-calorie, then those aren't necessarily low-calorie or not as low, like tagatose is higher than allulose, right? There's a lot of things to consider when you're replacing the sugar and what kind of formulations.
I do want to say that there are a lot of blends out there. I mean, companies are working very, very hard, ingredient companies are working very hard to come up with specific blends for different applications so ...
Which sugar substitutes are most effective for lowering water activity?
ZC: Yeah, great question. This is actually something that we talked about earlier in the table that Mary and I presented, where we were looking at comparing humectancy for these different alternative sweeteners, and which ones were the best according to the data?
MG: Yeah. Actually, what was best were the epimers of fructose, so fructose itself, but then also, allulose and tagatose. We could have those be able to reduce the water activity to like 0.20. It was really impressive, so long as they stayed in an amorphous state. I always like to say that a lot, but we could. We could get those to have a really good humectant effect, which means that they're going to bind with water really well, so it's going to have to be those epimers.
Do these sweeteners have the same impact when in syrup form?
ZC: Yeah. When we were going through our list of sugar substitutes before, we didn't quite get to the syrups, but there are definitely a few that are worth mentioning. What were some of the ones that you looked at in the lab?
MG: Yeah. There's a few, and mostly, the natural ones, so they aren't necessarily low-calorie, but like brown rice syrup, agave syrup, that little reduced calorie-wise. We also looked at tapioca syrup. Maltitol has the syrup as well, and these are actually really good to add. They're going to be in, I'm going to say it too many times, but does keep the form right, right?
It's not going to be able to crystallize with that, but there isn't added moisture content associated with those syrups, so you have to be able to account for that in your formulation, so just be aware that you are adding more moisture to that and it has to be accounted for, so either by baking, or cooking, or reducing, maybe some other source of water, but it is going to be a factor, so ...
ZC: Yeah, I would just add, just make sure that you watch out for any added moisture, and then maybe any adverse flavors, especially with some of them, so yeah. Just something else to keep in mind.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
