Webinars
Water Activity and pH: Working Together for Product Safety
When it comes to food product safety, the ultimate goal is often microbial stability. And in most cases, there are arguably two measurements that are the most important to stability, safety and effective hurdle technology strategy: water activity and pH.
Webinar Summary and Key Points
But which variable will give better control? Is it water activity? Is it pH? Is it a combination of the two? We’ll explore what water activity and pH are, how they affect microbial stability on their own, and how they work better together at controlling microbial contamination.
The first hurdle: what is water activity?
Water activity is a measure of the energy status of water in a system. From the First Law of Thermodynamics, we can derive the Gibbs Free Energy Equation, which provides information about the energy or chemical potential of any kind of species, which in this case would be water. The water activity in the sample is a direct correlation to the energy potential, which in turn is a direct correlation to the escaping tendency, or fugacity, of the water to the vapor phase
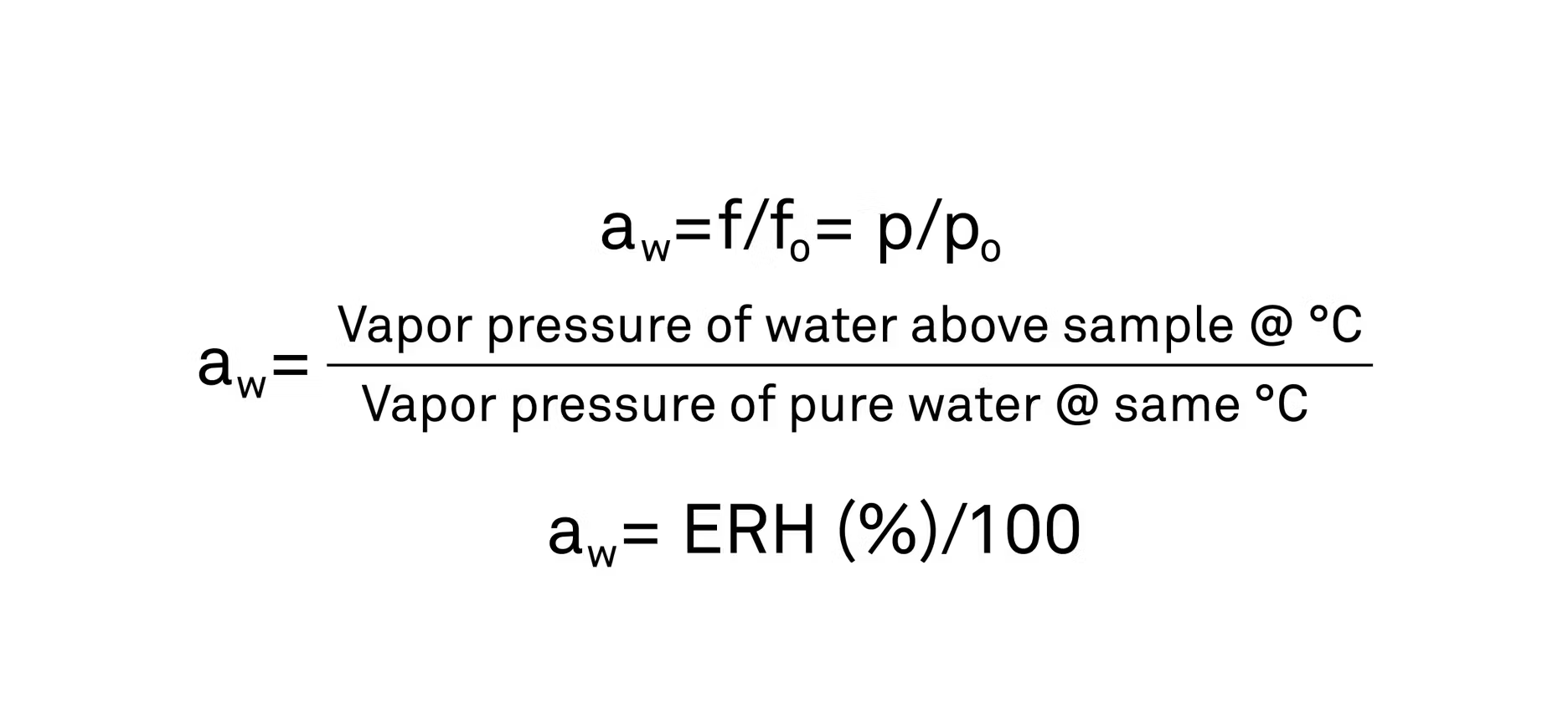
From a practical standpoint, to find the water activity of a product, you would place a sample in a sealed container, allowing the water to vaporize out of that sample into the headspace above it. That water vapor would create a pressure on the container which can be measured and calculated as (P), the partial pressure of the sample. Then, we can divide that number by the vapor pressure of pure water at the same temperature to give the final water activity in the sample on a scale of 0 (no water at all) to 1 ( pure water).
Though related, water activity is a different measurement from moisture content, which is a quantitative measure of the amount of water present in a sample, whereas water activity is the qualitative measure of the energy status of water in the system. While it’s useful to know how much water is present, microorganisms can only utilize water they can access.
Why Water Activity Matters
Each microorganism has a very specific water activity at which it will stop reproducing, brought on through the osmotic stress of halting the free flow of water through the organism’s semi-permeable barrier. By lowering the water activity of the system below this threshold, the microbe can be put into a dormant state and stop growing, thus achieving a stable and safe environment, as shown in the table below:
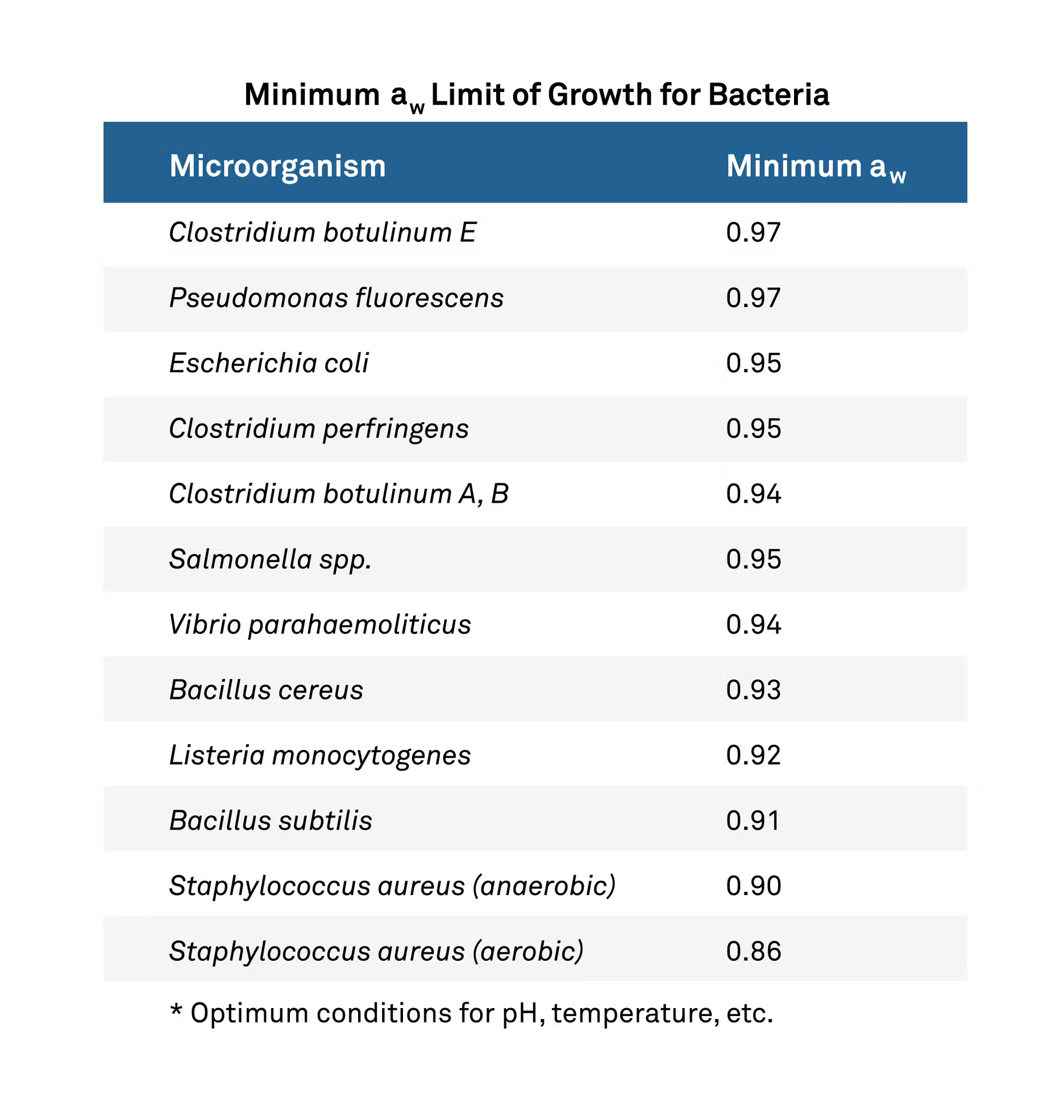
Water activity, however, is not a kill step. It merely prevents growth. Combining a lowered water activity with good manufacturing practices and a kill step will be the only way to completely remove the microorganisms from the product and keep them from returning and reproducing.
Measuring and tracking water activity in products is an excellent tool for assuring product safety and as a verification of control points in a good HACCP plan. Steady water activity ensures consistency in the manufacturing process and is a good marker that the end of processing has occurred.
The second hurdle: pH
In chemistry, pH is the scale by which we measure the relative acidity (or basicity) of a product. It works on a scale from 1-14, with 7 being the neutral point of distilled water. Numbers lower than 7 are more acidic (citric acid, acetic acid, etc.) and numbers higher than 7 are more alkaline or basic (baking soda, bleach, etc.).
A rough way to visually measure pH is through indication paper. The paper will change color and, by using the corresponding chart, will give a general representation of the pH of the product. A much more accurate measurement can be taken with photometrics by using a spectrophotometer, or by electrochemical measurement with a pH electrode.
Similar to water activity, we can establish minimum growth levels for different organisms using pH as the measurement.
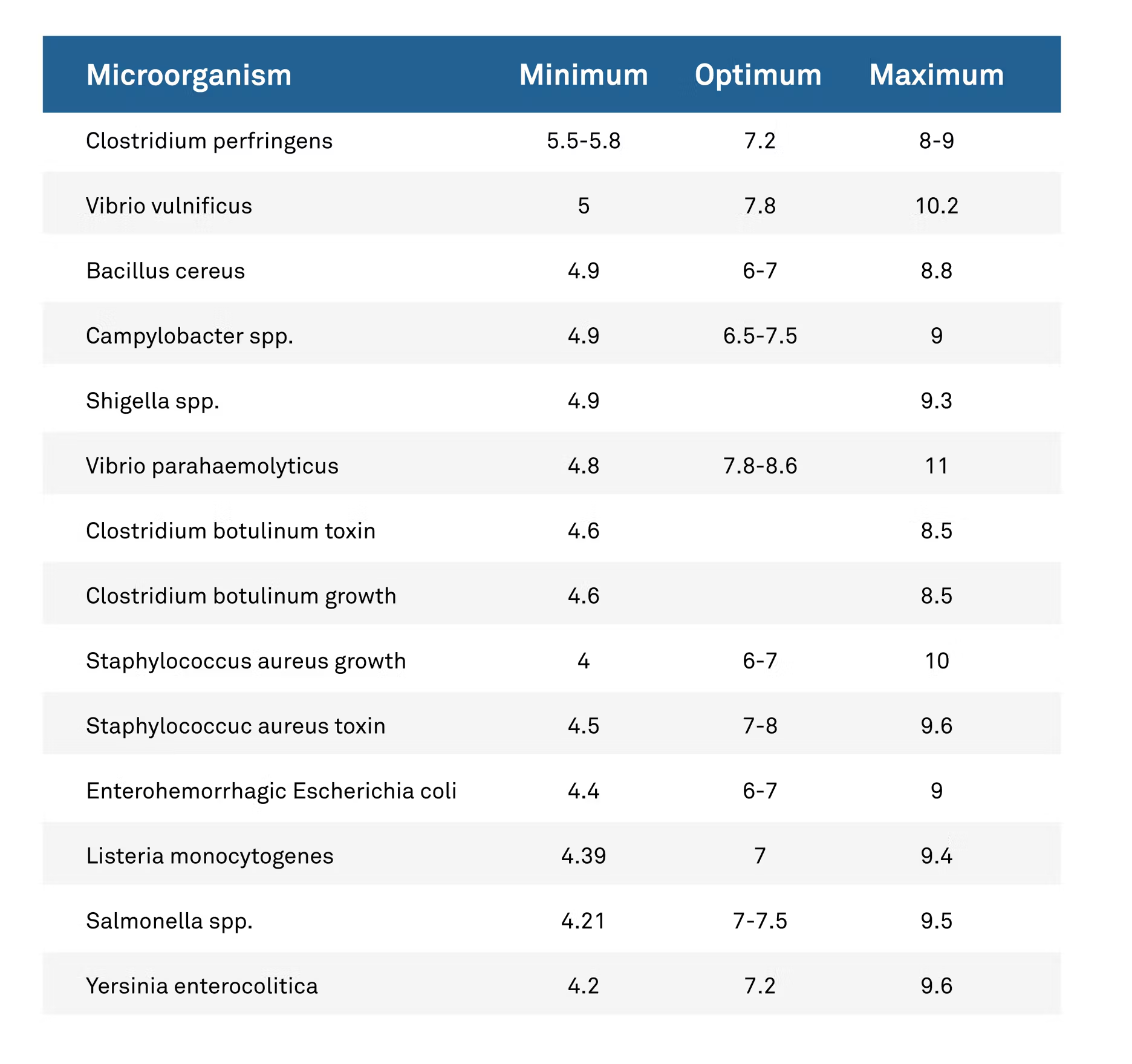
Recently, portions of the food code were changed from the previous minimum pH of 4.6 to include the particularly troublesome strains that can still reproduce down to a pH of 4.2.
Hurdle Technology: water activity and pH combined
While water activity alone, or pH alone, can inhibit bacterial growth, by combining their effects we can get more effective microorganism control with something called hurdle technology. Different factors such as temperature, water activity, acidity, preservatives, etc., can impact the growth of microbes, but by adding them together we can create a set of hurdles to prevent that growth.
In some cases, the whole of the solution is greater than the sum of its parts through the synergism of the agents changed, creating effective control at levels that would typically be considered unsafe for either pH or water activity alone. The table below shows this interaction between water activity levels and pH levels.
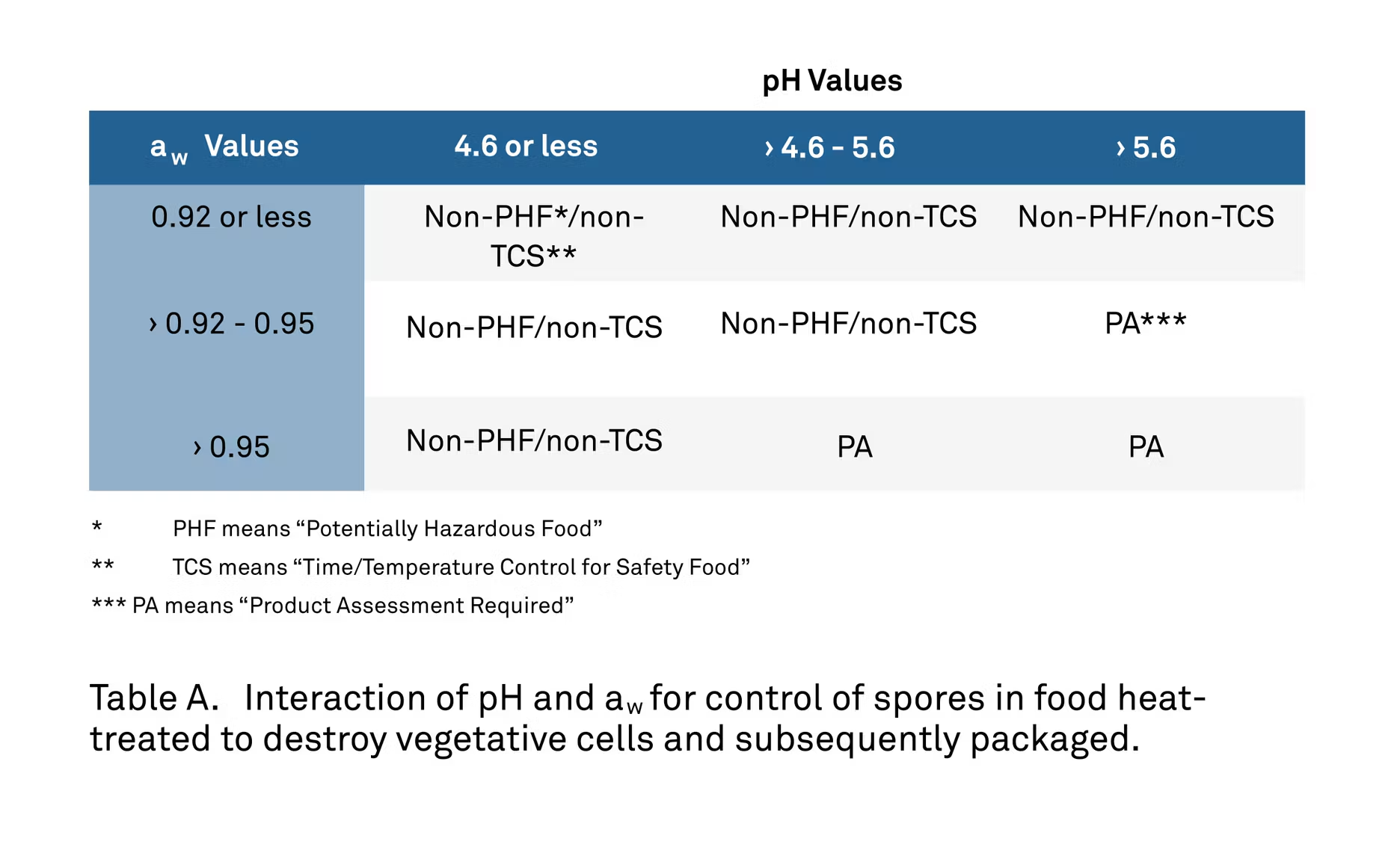
You’ll notice that the levels of water activity and pH are lower together than they would be to achieve the same results by themselves. Only at the highest levels of each will a product assessment need to be conducted, as denoted by PA.
How to change water activity and pH levels
How, then, do we control water activity in the product? The most common way is by drying or dehydrating the product to lower the water activity. As water is removed, the water activity goes down. But dehydration may not be feasible for all products, and this is when humectants are used.
Humectants are materials that lower water activity by binding up the usable water in the food without removing more water content. The most common materials used are some sugars, like high fructose corn syrup, or salt. There are others such as sorbitol, maltodextrin, glycerin, and even some starches, though the solubility of these ingredients becomes the limiting factor.
Tools are available for purchase from METER Group that help calculate how much of any one humectant is needed to achieve a certain water activity level. This will give a good point to start measuring and testing additives and their effects.
And how do we control pH levels? The common way here is through the addition of different acids (acetic, citric, lactic, etc.). Sometimes it’s through the addition of naturally acidic ingredients themselves. Take spaghetti sauce, for instance. The tomatoes lower the pH of the sauce, making it acidic enough to be stable.
Another way to control pH is through the fermentation process. Preferential bacteria grow, and in this growth product lactic acid, which then lowers the pH of the food, preventing the growth of other types of organisms. Examples of fermentation would be pickles, sauerkraut, olives, etc.
While both of these factors work well alone, they are even more effective when used together. By having a HACCP plan in place with water activity levels and pH levels as control points, routine testing can ensure that the manufacturing process is consistent and, ultimately, that the product is safe.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
