Webinars
Why Powders Misbehave
Caking, clumping, solidifying in storage, sticking to machinery — if you work with powders, you’ve seen the obvious messes and misbehaviors
But you may not have seen the dangerous mischief powder can make elsewhere. These less visible, lesser-known issues can be a big deal – like health hazards and product recalls. Ignore them at your own risk.
Join Mary Galloway, head of the METER Food R&D Lab, and Dr. Zachary Cartwright, lead food scientist, as they present new research findings and break down the many reasons powders misbehave.
You’ll learn:
- How many companies accidentally misrepresent their products’ functional food benefits
- The microbial risks of low-moisture foods and the dangerous misconceptions about them
- The multitude of factors that influence powder stability and which ones are most important to watch
- How to pinpoint where powder problems will happen and how to prevent them before they begin
About the presenters
Mary Galloway is head of the METER Food Research & Development Lab. She specializes in using and testing instruments that measure water activity and its influence on physical properties. She has worked with dozens of the world’s largest and most successful food brands to solve moisture-related product issues.
Dr. Zachary Cartwright is lead food scientist at METER Group. He holds a PhD in food science from Washington State University and a bachelor’s degree in biochemistry from New Mexico State University. He is an expert in isotherm analysis and the use of the Vapor Sorption Analyzer (VSA).
Transcript, edited for clarity
Zachary:
Hi everyone. Welcome to Why Powders Misbehave. My name's Zachary Cartwright.
Mary:
And I'm Mary Galloway.
Zachary:
Let’s start with a brief outline of what you can expect today. We're going to talk about what powders are, try to give you a definition, and then we're going to focus mainly on three different aspects of powders. We're going to look at physical stability like caking and clumping and loss of flowability. Mary has some lab data that she will share with us, and then we'll move into chemical stability, thinking about rancidity or browning reactions. I believe you also have some research for us.
Mary:
I do. We did a study in the lab on degradation of vitamin C.
Zachary:
Finally we'll look at some microbial stability. Even though this is a low water activity environment, there are still some microbial concerns, and I believe you were just working on an article or quoted in an article that we'll be focusing on.
Powders 101: Foundations and definitions
Zachary:
Mary, what is a powder? How would you define a powder?
Mary:
Well, most people know what a powder is in theory because we find them in so many places. When we were discussing this before, market mattered. When we were talking about the category of powders, we could have spices, ingredients. The pharmaceutical industry also uses the word for excipients and APIs, and they serve a lot of different functions.
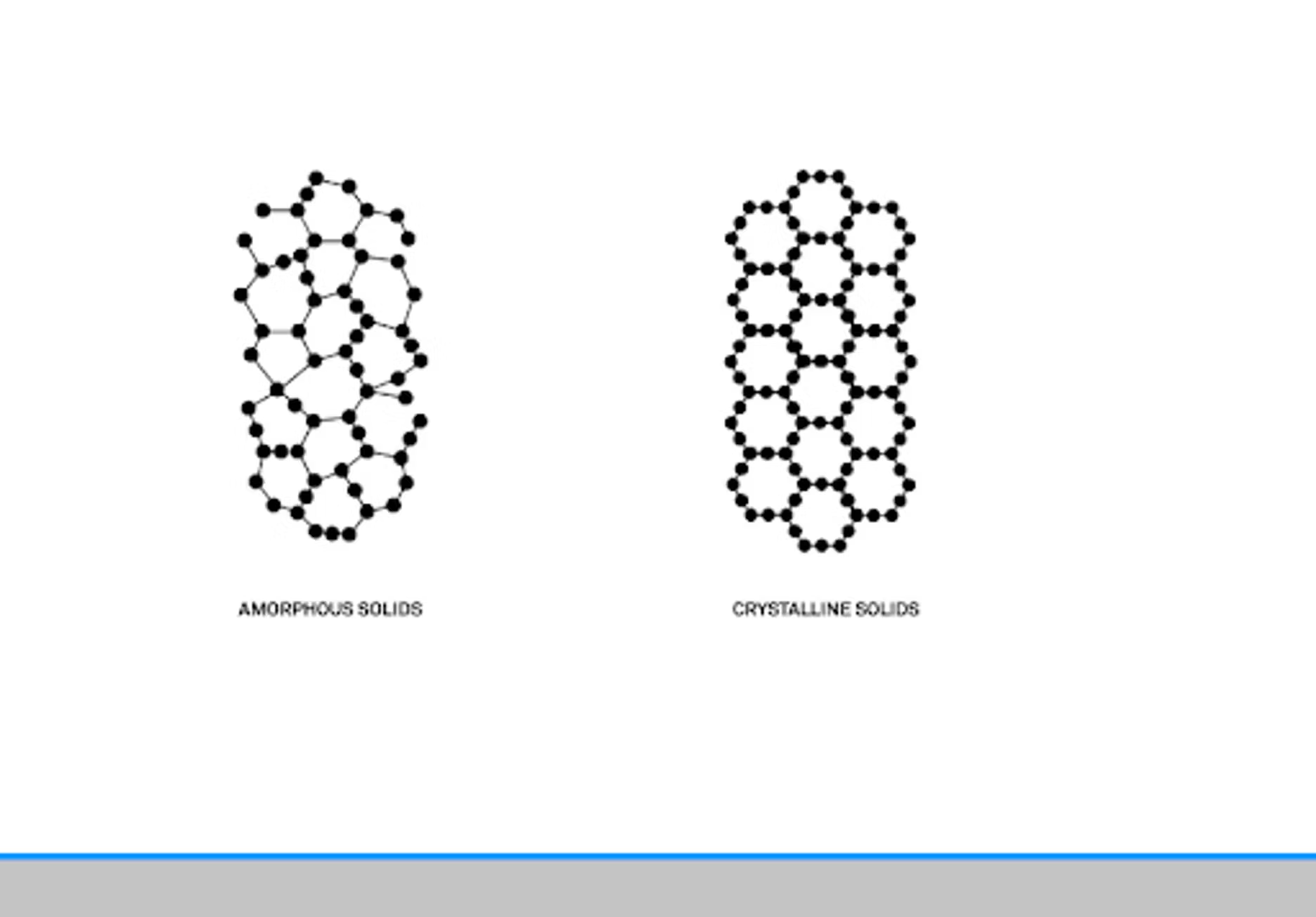
Basically, a powder is a small granular product. We found a good definition in a paper from Bandari from 2017. To paraphrase, it talks about structure as being the main definition of a powder. Amorphous, crystalline, or combinations of those two together. How those two interact, and the particle size, will affect the functionality, the application, and the production of the powder.
That sums up why powders are such a huge market and why they're so difficult — because they span so many different markets and functional groups.
Zachary:
We've done a previous webinar on powders where we dove a little deeper into looking at amorphous and crystalline. Basically from a molecular structure there are some key differences. A crystalline structure will have a repeating structure that's well defined. That's something you can see on a molecular level. Even visually, you can see these differences in powders, and we have some figures for that.
Last time we talked about mixing these powders and looking at some of the combined effects. If you are interested in looking more at that, you can look back at our previous webinar. We also talked about particle size, and I want to hit on that more. How does particle size affect some of the characteristics of powders?
Mary:
Particle size is a major impact for characteristics and powders and why they can be so difficult. When you have small particle sizes, then you can get bridging between those particles that you don't anticipate, and then you can start getting them sticky and they have agglomeration and things like that.
There are a few things besides just general particle size. Particle shape is also a factor too. There's been some research too, when you mix crystalline powders together, you'll have what we call deliquescence, which is basically going from a solid to a liquid state earlier than you would anticipate either of the individual powders doing. The reason is because of having different sizes of particles.
Whenever you get these places of contact, you can start having bridging and problems. Crystalline powders can be particularly tricky because as you said, they have a very ordered structure, and that means the moisture only adheres essentially to the outside of the structure. It's only a surface interaction as opposed to with an amorphous powder, there are a lot of crevices and irregular shapes and sizes and water can more easily bind with the amorphous. That does make them functionally different, but it also impacts when you have to use them as a formulator.
Zachary: Last time we talked, we looked at the five stages of caking. There are multiple steps to getting to agglomeration and then eventually to liquefaction.
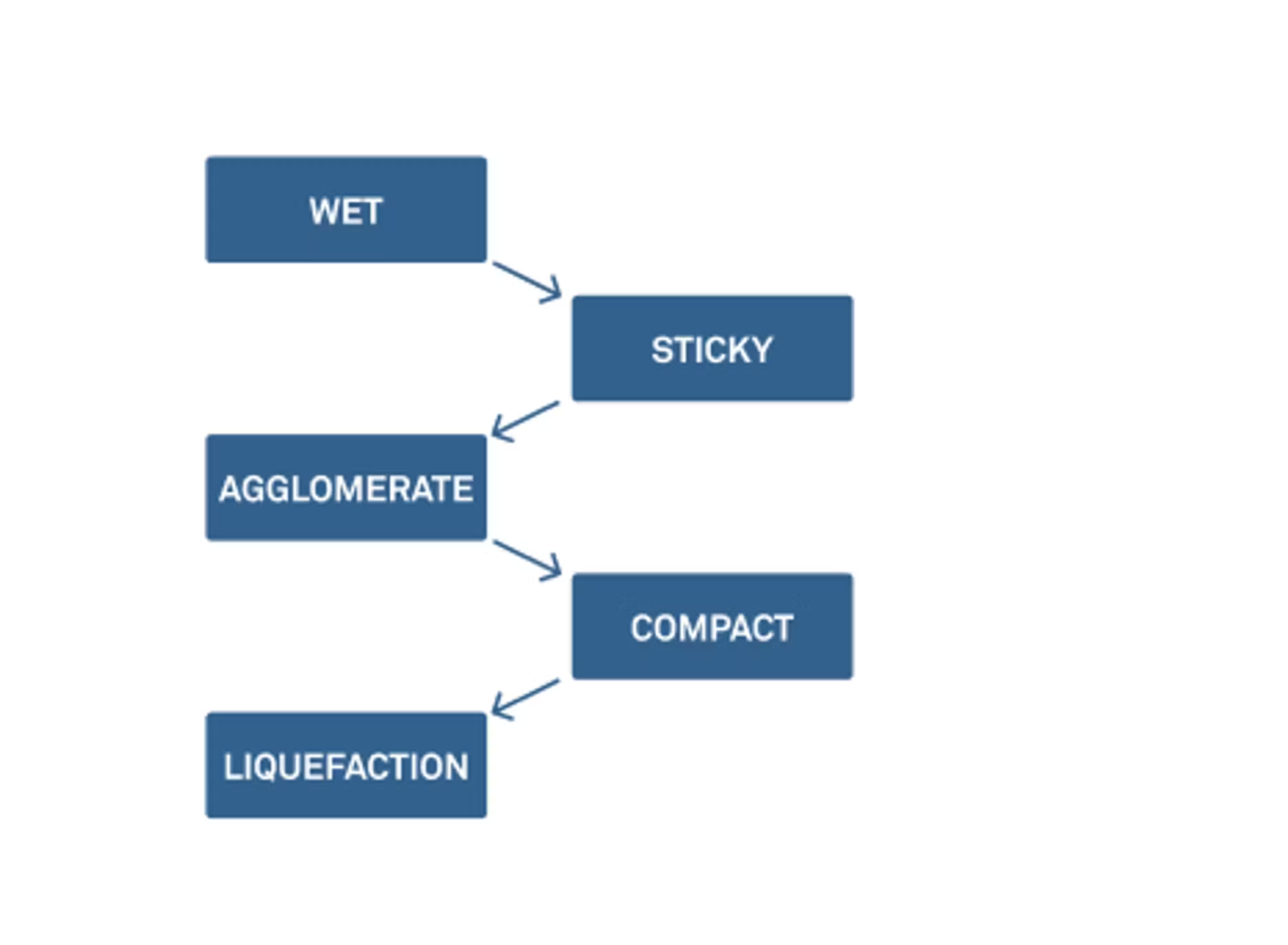
We don't necessarily need to go through them today, but I do want to point out that caking and clumping can start to occur at an early stage. One way to control this is by looking at the moisture and the water activity of these powders. We do this in most of our webinars, but it's always helpful to define what moisture content is and water activity and talk about how we can use these things together to think about physical changes and chemical and microbial stability.
Let's start by looking at moisture content and water activity. I know you have a pretty good graphic for this and a good definition. How do you differentiate these two different measurements?
Mary:
For some of the people we speak to, water activity is a new concept, and most people are very familiar with moisture content. I like to separate them and say that there are two water measurements we can make. There's one where we look at the amount of water, or the moisture content. The other is to look at the energy of the water – what is the water able to do? We measure those in completely different ways.
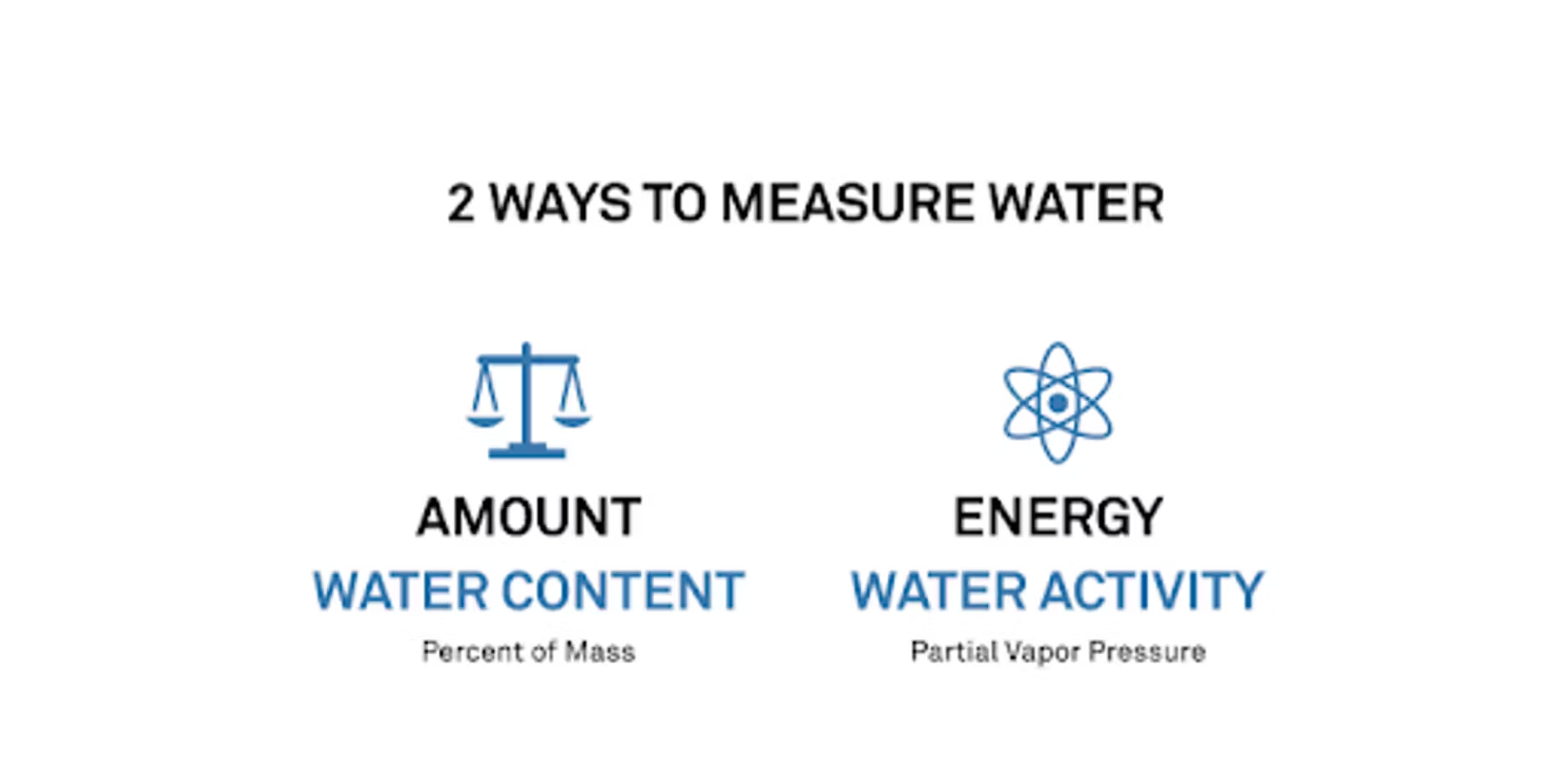
When we look at moisture content, that's a mass percent, so we're just looking at a weight. But when we're looking at water activity, we're actually measuring what we call a vapor pressure, so that's similar to what the humidity is coming off of a sample.
If you want to look at some of our research and some of our other webinars, we talk about water activity. It might be helpful to consider what we're referring to is basically an equilibrated humidity the sample is putting off, and that might help people understand these two things better. Also how some external and ambient conditions can affect their product, which is also something to note and to monitor as well.
Zachary:
Good point. I still see it misdefined pretty often that water activity is water availability, and that's not exactly the right way. Water activity is a thermodynamic principle. It really is the energy of that water, and it's important to know because the energy of the water can be used for a chemical reaction or texture change or something else. It's good to hone in on that point. We are looking at the energy of the water with water activity.
We have clients that come to us all the time who have a good record of moisture content, but they have a really hard time getting a precise measurement. Because they can't be precise with moisture content, it's hard to connect some of the problems they're dealing with to moisture content. Moisture content alone isn't going to give you all the information you need, especially for powders.
By combining water activity and moisture content, we can look at the isotherm, and this is something that we always talk a lot about, but it's because it's a unique way to look at the water in these products and get a complete picture of how the water is behaving in that product. How do you take an isotherm? How do you look at that shape and correlate it to different characteristics of a powder?
Mary:
Some of the ways we use isotherms is to define a critical point, a critical water activity where we're going to start seeing texture changes and other changes within the structure of the product. Basically, at what point does it start to change and take a lot more moisture? Generally if we're talking about a powder, that is the point where you're going to start seeing caking and clumping. If we have some other product like a snack, it can start getting soft, so those are critical points to find out. We can also look at the slope or the shape of the isotherm itself too and be able to identify the structure like we talked about amorphous versus crystalline. We can look at that. We can also by exposing these samples to humid air and basically in real time see how they behave, we can get a lot of great information about that product that can be useful for a client.
Zachary:
It's important to point out that we have a unique method called a dynamic dewpoint isotherm, and this is the best way to get a high resolution graph or picture of how the water's behaving. There are other methods that we'll talk about later, but by using that dynamic dewpoint isotherm and by using the vapor absorption analyzer, this is the best way to characterize the water and then to look at some of the factors that we're going to consider starting with physical stability. In this next section, let's talk about physical stability and what that means for powders.
Mary:
All right.
Characterizing the physical stability of powders
Zachary:
Let's dive deeper into physical stability. When we think about physical stability and powders, there are three major factors that we want to consider; moisture, temperature and time. I'll let you dive into these and add on as needed.
Mary:
For moisture, basically, if we have more availability of moisture, we're going to have more processes to change, more structure to change. When you were talking about the DDI, my first thought was looking at that in terms of a spray dried milk powder where we're going from a glassy state to a rubbery state.
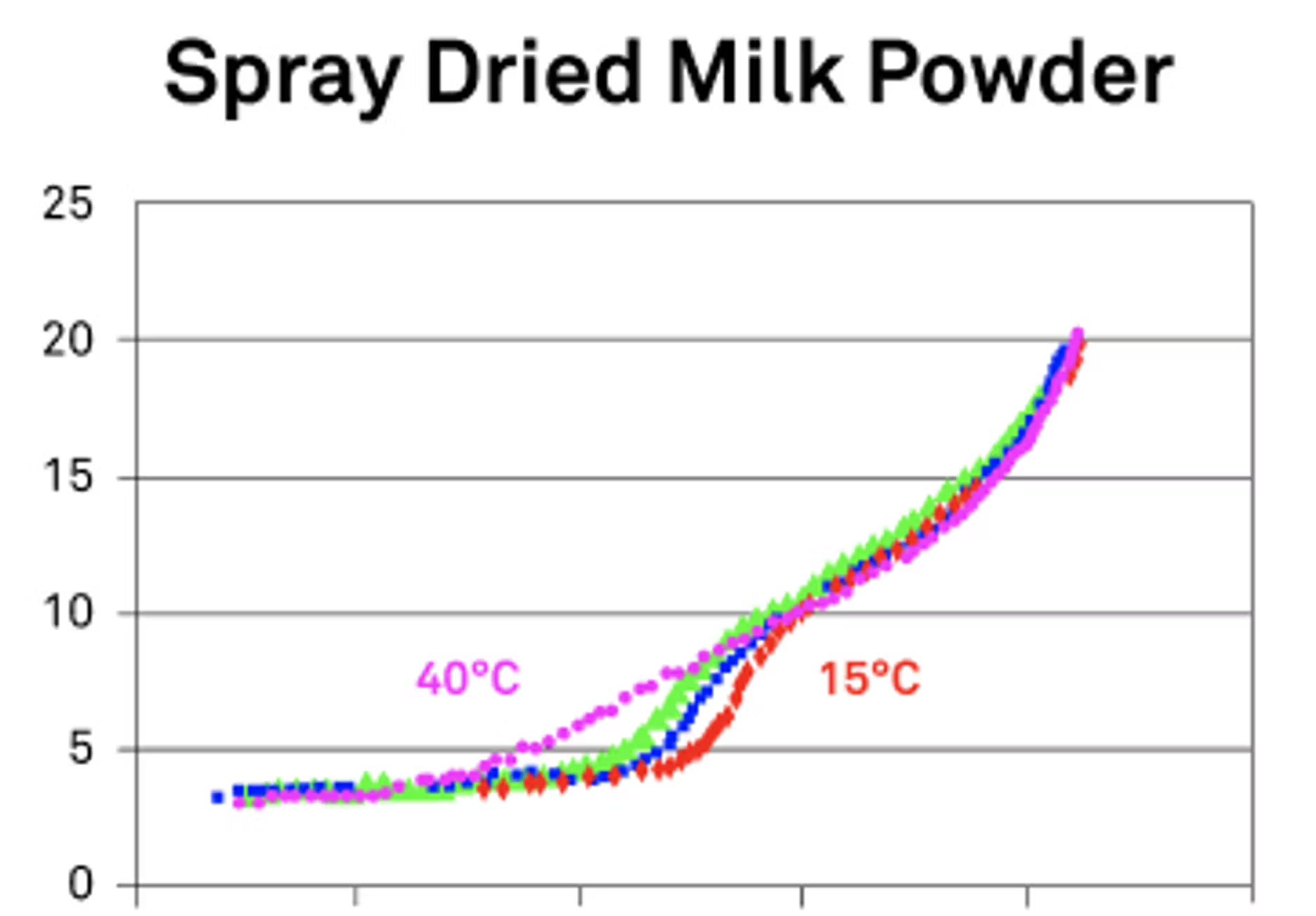
Now, if we did a different more traditional style of isotherm, you actually miss that transition because it essentially jumps over it. You normally hold things at certain humidities and see what happens. But in the DDI, it's the real-time process and you can actually see these transitions happening. This is what I specifically mean when I'm talking about using the isotherms to be able to see your realtime data and when those critical points start to happen. Moisture is a major impact for those processes because water tends to speed things up.
It can be a solvent, it can be a reactant, and it can actually even be a buffer into the chemical reactions. Sometimes the roles change as the process goes along or as more moisture is added to that process, and then you can see different reaction rates to actually change. Besides moisture, we also want to look at temperature. We have a graphic that we like to show because it's a great way to express how that critical point that we've been mentioning will change as we're adding heat or increasing temperature to a product. What happens, and it does make sense when you think about it, is that that change happens faster. You're adding energy into the system, and that system it's flowing faster. You'll get these changes happen at what would be lower water activities or faster in that process.
The last thing is time. If you give a process enough time, it's going to change. Even if you could hold the other things just static temperature and moisture, if you gave it enough time, it would happen. I was just thinking the other day about this example. You've got old glass windows, and if you were to measure the top and the bottom of very old glass windows, you would know that the bottom is thicker than the top and that's because they've had a long time to flow. That's this idea of if you give something a process long enough, it's going to come to completion. All three of those factors will play into physical stability.
Zachary:
It's also important to note, depending on your question, there are different methods or different types of isotherms that you can use. Going back to the dynamic dewpoint isotherm, you can use this to find that critical point and understand exactly at what water activity or at what relative humidity and temperature combination will cause that effect. Then once you know where that critical point is, then you can also do a DVS test, a dynamic vapor absorption test to ask the question about time.
Going back to your window example, how long is it going to take to actually reach this critical point under a certain condition? We have a way to answer that. We have our vapor absorption analyzer that allows you to do both of these methods, which is unique and really the only instrument capable of doing both of those. If you have a problem with physical stability, having access to both those types of tests can be effective.
Now let's move into your spice mixing project. What was this project? What was the goal of it and what did you learn from it?
Mary:
As we know, moisture will move because we have differences in water activities. The question is how much does it move? Can we predict that and how accurate is the prediction? We do have a tool. There are definitely equations out there that try to model that interaction between products. What we did for our project was essentially that I took six different blends, some spices, some we did a maltodextrin in sorbitol, corn starch and onion salt, and then some spices, sage, oregano and cumin. We put them together. First, we forced all of them to have a very specific water activity. Then we also ran isotherms on all of those ingredients because that is a major factor, not just the starting water activity or a mass ratio in the blood, but what is the characteristics of that isotherm of how does it behave in the presence of moisture.
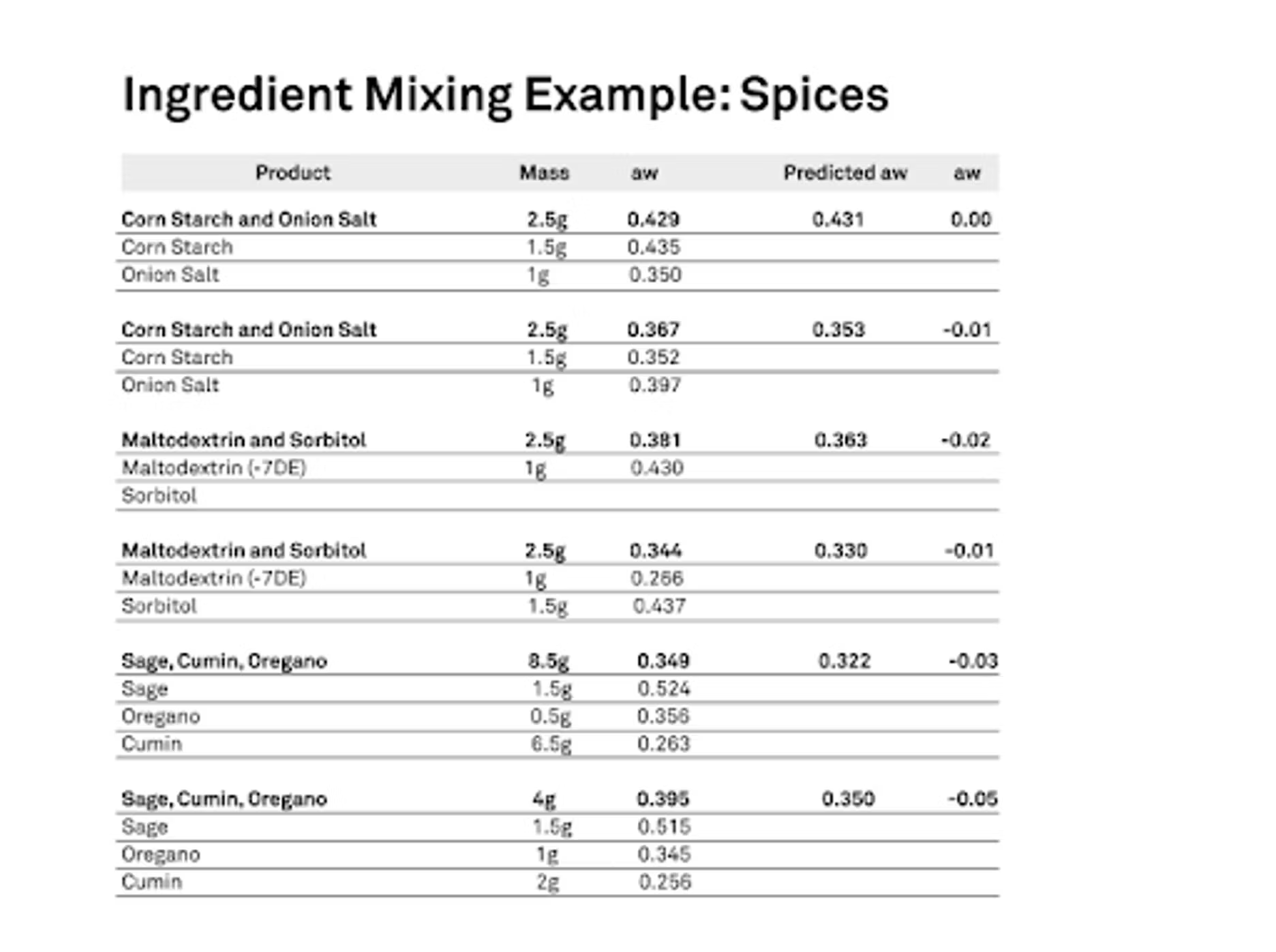
We want to know how they take up or don't or however that might be. We need to know what that information is for that product so we can do a good predictive model. We did this, and then we mixed them together in known mass ratios. Then we measured to see exactly what the water activity was after we gave them time to equilibrate. Then we also did the prediction, and they were very good. Basically, I'm showing the combinations that we did, the top one there, corn starch and onion salt. We mixed one and a half grams of corn starch, and it started at a water activity of 0.435. The onion salt, we did it as a gram, but we had it at a lower water activity. You'll see there that it started at 0.35 water activity, and then we blended this and our blend actual water activity was 0.429.
When we ran the predicted model that took the isotherms into account, the initial mass and the initial water activities, we actually predicted that the final water activity would be 0.431, so extremely, extremely close. These worked well. They have fine particle sizes actually, so they have a lot of contacts, so you're going to get a quicker equilibration. That wasn't too much of a surprise, but it was nice that it worked so well. You can see with some of our other examples that we ran very well. We also did maltodextrin and sorbitol. We varied the amounts, we varied where they started. One was higher than the other one, and then we swapped it. We tried to do various combinations to just test it out a little bit. Then we also did the spices at the bottom; the sage, cumin and oregano.
Those worked quite well. Our worst case scenario in the setup was the very last example. — maybe I shouldn't say that, but the scientist in me says I need to. You’ll see that our predicted was 0.35 water activity where our actual was 0.395. It was about 0.05 lower. I just wanted to talk about how this works and then the comparisons we did.
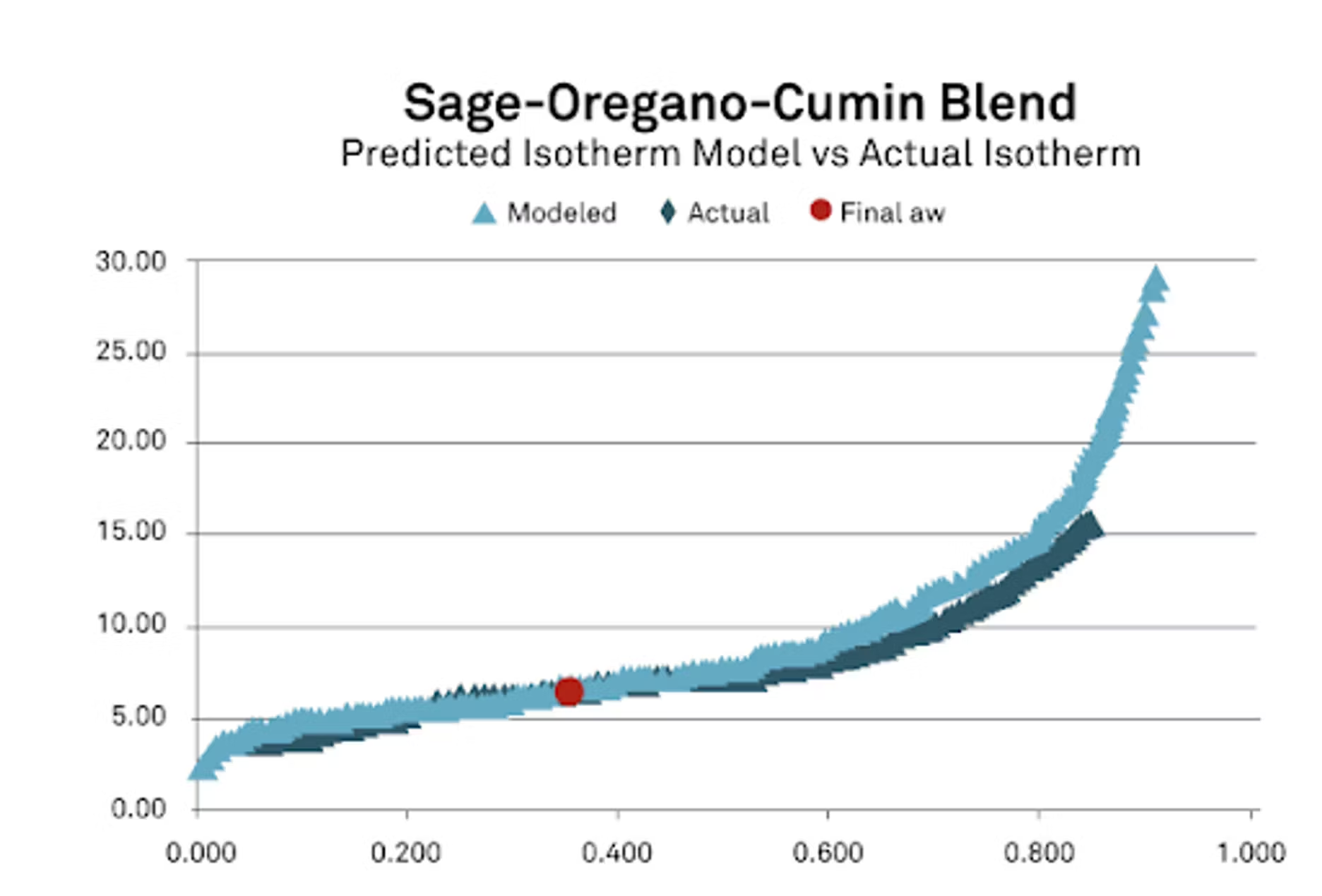
You'll see here all of the isotherms for the sage, the cumin, the oregano, a combined model, so where we put those all together. I also wanted to show where we started. This is where we started with all of the ingredients with the initial water activity, with the initial moisture content based off of the isotherm and the mass ratios. Once we put it all in there, you can see there that we got a final water activity of 0.349.
Now for this, what's important is we're doing this mathematically, so we want to make sure that we have a good representation, a good mathematical equation and coefficients for each of our ingredients. Once we got that, we got our prediction, it was pretty close, I was pretty happy with that. Particle size perhaps with spices, maybe not having as good a contact, it's possible that once we let them be together longer, we might have had a slightly different result. But I was very pleased with the result that we got. I also wanted to take this and look at it the other way.
We modeled the isotherm as I'm showing here in the red trace, but then I wanted to also compare that with the real isotherm because after we mixed this blend, we actually ran an isotherm on it to see what we could do. From there you can see the differences between the actual isotherm and the modeled isotherm, and they match up very, very well and especially in the area of interest that we're looking at. If we're looking around for spices, maybe 0.2 to 0.4, it's generally where they live in the water activity range. You'll notice we have a really nice fit. I feel very, very good about our data in our study. This, like I said, is the worst case that we had. The rest of them actually had much better results.
Zachary:
I just want to take a step back and think about the real world application of this. I talk to scientists all the time who are under a lot of pressure to get new products out as fast as they can. If you use this type of modeling for a dry ingredient mix, this is a fast way to get lots of insights about that final product before you even make it. It does take a little time to build up a library and have isotherms for each ingredient, but once you do that, then you can sit at your computer and very quickly understand what the equilibrium water activity will be. With our new program in the Moisture Analysis Toolkit that comes with our vapor absorption analyzer, the toolkit software does all of the work for you. You mentioned those equations and there are equations in the background, but instead of having to build your own spreadsheet or do this all yourself, all of that labor is being done for you and it makes it easy to know what the equilibrium water activity will be.
Now you can also get the coefficients for that model that you mentioned. Using those coefficients, you can start to ask questions about what is the predicted shelf life or how long will it take to reach a critical water activity or what type of packaging should I use? There's a lot that you can do if you take the time to look at these isotherms and understand exactly, how can I use this data to answer lots of different questions? I just wanted to point that out that the graphs that you're showing do come from the Moisture Analysis Toolkit software. This is something that a lot of our clients, whether they're making nutritional supplements or we work with some of the largest spice producers in the country, use these equations and these tools to speed up their production.
Mary:
Good point. I also wanted to add that once you have the isotherms, I can rerun this prediction over and over. I can change any part of it and rerun it in just moments. As an example, I could change the mass ratio if we find that we don't particularly like this blend or we don't like the flowability of it or something, or maybe the flavor isn't good. Maybe it's got too much cumin in it or something. You could adjust that recipe, and you can just do it in the software. Or let's say as an example, we've talked in prior webinars about the seasonality, how that can change your incoming ingredient.
Water activity tends to be higher in the summer and lower in the winter. It's important to monitor what you're getting when it arrives so you don't get a surprise and moisture coming into your product that you didn't anticipate or want. That's something you could change here. You could change the initial water activity of any of these products and then just rerun the prediction. Once you get that information, you can do a lot with manipulating it.
Zachary:
Well, that was all mainly related to physical stability. Now let's jump to chemical stability and talk about how we can use water activity to inform or to understand chemical stability better.
Characterizing the chemical stability of powders
Zachary:
Moving on to chemical stability, I want to look back at our stability diagram that we had up before.
The reason that this is important is because you have to think about whether your powder delivers the health benefits that were promised. The vitamins, are they there that you promised or has there been some type of chemical change that you need to be aware of? If you look at that stability diagram, there are different points in that diagram where degradation rates or reaction rates are going to change, for example, around 0.6 water activity, you might have an increase in browning reactions. At very low water activities, this is where we see lipid oxidation start to increase.
You just need to be aware of how water activity is affecting reaction rates and which reactions are related to an end of shelf life. I know that you recently worked on a vitamin C experiment and looking at how it relates to what activity. Can you explain this experiment and what you found?
Mary:
Chemical reaction rates are a little more complicated to track, but if you can track them and that is doable. To be able to track the reaction rates, we can use that information to predict a shelf life and a timeline essentially to when they've reached a point where we'd consider the shelf life ended. Our study was on ascorbic acid, and basically we exposed it to two different water activities and three different temperatures. Then we tracked how it degraded using UV-Vis, and we were able to calculate those rates.
One of the parts that's critical about this is we were using the Arrhenius equation, which is very common to use for this type of reaction. Basically you're going to connect a rate to a temperature and an energy. We already know that we can relate energy to water activity specifically.
We run a study where we're starting at time zero, and then over a period of several days to weeks we've gone and exposed that ascorbic acid to a specific community and temperature, and then we watch it change, and then we can graph it. This is our study. Essentially we would like to know how temperature and water activity are going to affect the degradation rate. We've got these time plots here that we're showing the graph. We have our water activities at 0.76 and 0.948, and the temperatures that we used, and we are running these as an accelerated study. We have 30 degrees C, 40 degrees and 50 degrees C. Essentially we'll put this into the calculator and we use the Arrhenius equation to help draw the information and correlate everything. Once we get all our data and our study data in, then we can tell the program specifically what our interest is.
What temperature are we interested in? What water activity are we interested in? Then we also need to define in the study where we're going to end shelf life. What percent is left? In our case, what we did was we decided that 75% of the vitamin C left would be the end. We would essentially lose 25% of our vitamin C, and then we would call that the end of shelf life. If you're a formulator or a manufacturer, if you have a vitamin like you were talking about, you have claims for so much vitamin or the potency of your product. You would base whatever ended shelf life on that value.
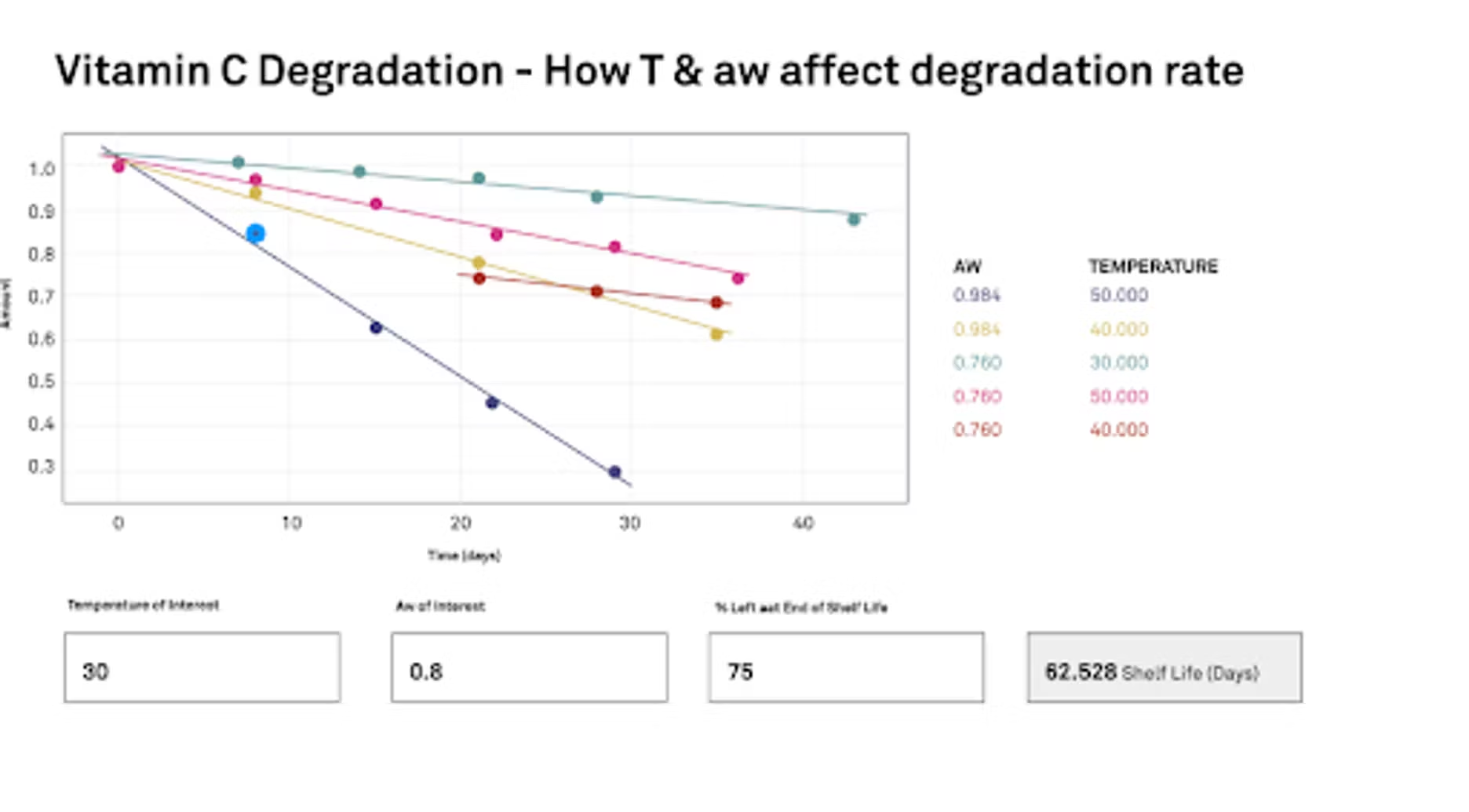
Once we can put all that information in, then it can calculate a shelf life. In our case here with our ascorbic acid, if we were interested at 30 degrees C, and we were going to have it around 0.8 water activity, which is pretty high, but a steamy bathroom could get there. Which is relevant to about 80% relative humidity. Like I said before, 75% is going to be the end. We would have 62 days before this ascorbic acid was no longer potent enough.
That's basically how chemical stability works. We just need to track it, and if you're able to measure that, then you can do this study.
Zachary:
Again, this is another tool in the Moisture Analysis Toolkit, specifically for chemical stability. Even though it can take some time to gather data, once you have that data collected, it's easy to graph in the toolkit. You just showed you can put in a starting water activity, a temperature of interest and then define what's ending your shelf life and quickly get calculations. Even though it may take some time to set up a study, and these are studies that we can do or we can also guide clients through using humidity chambers, once that data has been collected, there's a lot of flexibility with the calculations and a lot of insights that you can get. We were looking specifically at vitamin C in your case, but this could be for anything that we can measure, anything that we can assign a value to.
Mary:
It goes both directions. It could be in this case that this is the degradation rate, but you could do an increase of something as maybe a browning reaction or something like that increased over time, and then that's what ended shelf life. It was maybe a specific color value. It doesn't matter as long as you can measure it. If there's some way you can actually track that change, then you can turn it into a rate and you can get plots like this.
Zachary:
There's so many nutritional supplements now and so many things are in powdered form that using this type of tool could help them understand what their water activity target is or shelf life conditions or what does end their shelf life. What percent of losing a certain vitamin or how can they make that claim that's on their label? How do they know for sure? This is a good way to do that.
Mary:
Again, the example before you could change your interest, you could change your interest. You could change your temperature and your water activity of interest or even how much you want at the end. All of that is just easily to be changed and just rerun the prediction.
Characterizing the microbial stability of powders
Zachary:
Finally, microbial stability. A lot of powders are going to have a low water activity, and sometimes people don't recognize that there can still be a safety concern there. I mean, powders could have a microorganism of concern, and it may be safe, but it's not necessarily sterile. Once you rehydrate a powder, if there is a spore there or a microorganism of concern, once it's rehydrated, this is when we can start to see some safety issues. I understand that you recently contributed to an article, and I was hoping that you could talk about what was in that article.
Mary:
You said it exactly. There is an idea that if you have a low moisture food that you don't have to worry about microbial growth and you don't need to know about water activity but there've been many, actually sadly recalls on low moisture foods and where they have had outbreaks of E coli and salmonella like in peanut butter or flour, things like that, baby formula. It's disheartening. That is exactly the case is that water activity is an excellent way to limit microbial growth. If you have a water activity below 0.6, nothing will grow. That gives a false sense of safety. My product is low, I don't need to worry about it, but water activity is not a kill step as just what you were saying, which means they still live, they're still in stasis.
If they are exposed to a higher humidity or higher water activity, environment, if you put flour into cookie dough, which is what you would want to do with it, now, you've created an environment where those microorganisms can now grow and proliferate and you can see where the salmonella or E coli or whatever becomes dangerous to the public. This is actually a large topic. When I went to a conference on food protection recently, they did discuss this quite a lot, had many sessions on it, and right now there's a lot of research out there too on what can we do to possibly sterilize or pasteurize these low moisture foods. The research is being done out there, and we know several researchers, and I know you do as well, that are working on this very actively to try to think of and come up with these ways that we can to not have any microbial growth or these outbreaks that are happening that we can pasteurize or sterilize these low moisture foods.
Zachary:
One of those researchers we recently talked with is Dr. Jennifer Acuff, and she's looking at low moisture foods and environments that they're produced in and thinking of different ways or sanitation techniques or how can we prevent food-borne pathogens from being even in low moisture foods. We recently recorded that podcast with her, and that's something that she discussed. From my point of view, staying on top of your sanitation and making sure your environment is as clean as possible. This is something that we also talked recently to Dr. Minto Michael at Washington State University about again, looking at microbiology and understanding there are different temperature, time combinations and water activity, but like you mentioned, you can't heat every type of food because you're going to cause some structural change.
There may be other ways, microwave or high pressure processing or something else that we can use in combination with thinking about water activity to make sure that these foods are as safe as possible. If you'd like to, you can listen to the podcast with Dr. Acuff or Dr. Michael to learn more about food safety.
Mary:
I also thought of how water activity actually plays a part when you're doing your pasteurization step. Even if you are able to do that, let's say if it's beef jerky or something like that, there is a time temperature combination and humidity as well to make those effective. That's the other part of it is that if you're able to do that, are you creating an environment to actually kill the microorganism that you're interested in? If you do the right time and temperature but your humidity is low, you're not as effective in sterilization as well. It's all these factors for sure.
Recap and recommendations
Zachary:
As a brief recap:
- Today we looked at powders, we tried to define them.
- We looked at physical, chemical and microbial stability.
- We talked about some of the AQUALAB products that we used to research and inform what we talked about today.
One thing that I wanted to mention here is that even though we make solutions that measure final products or measure ingredients, we also have an inline solution called SKALA Dry that can really help with spray dried products.
If you're having any difficulties making a consistent product as the temperature, as the seasons change, SKALA Dry can automatically help you hit the right water activity for that product.
Also, please check out the podcast that we mentioned, Water In Food. We also have a YouTube channel. Please make sure to listen and subscribe.
At this point, let’s open the floor and take some questions.
1. Is there a way to measure or track the hygroscopicity of the powders I work with?
Zachary:
Great question. When it comes to hygroscopicity, we can look at the shape of the isotherm curve. This is something that we brought up earlier, and we can probably bring up back now, but you're looking at the slope of the curves.
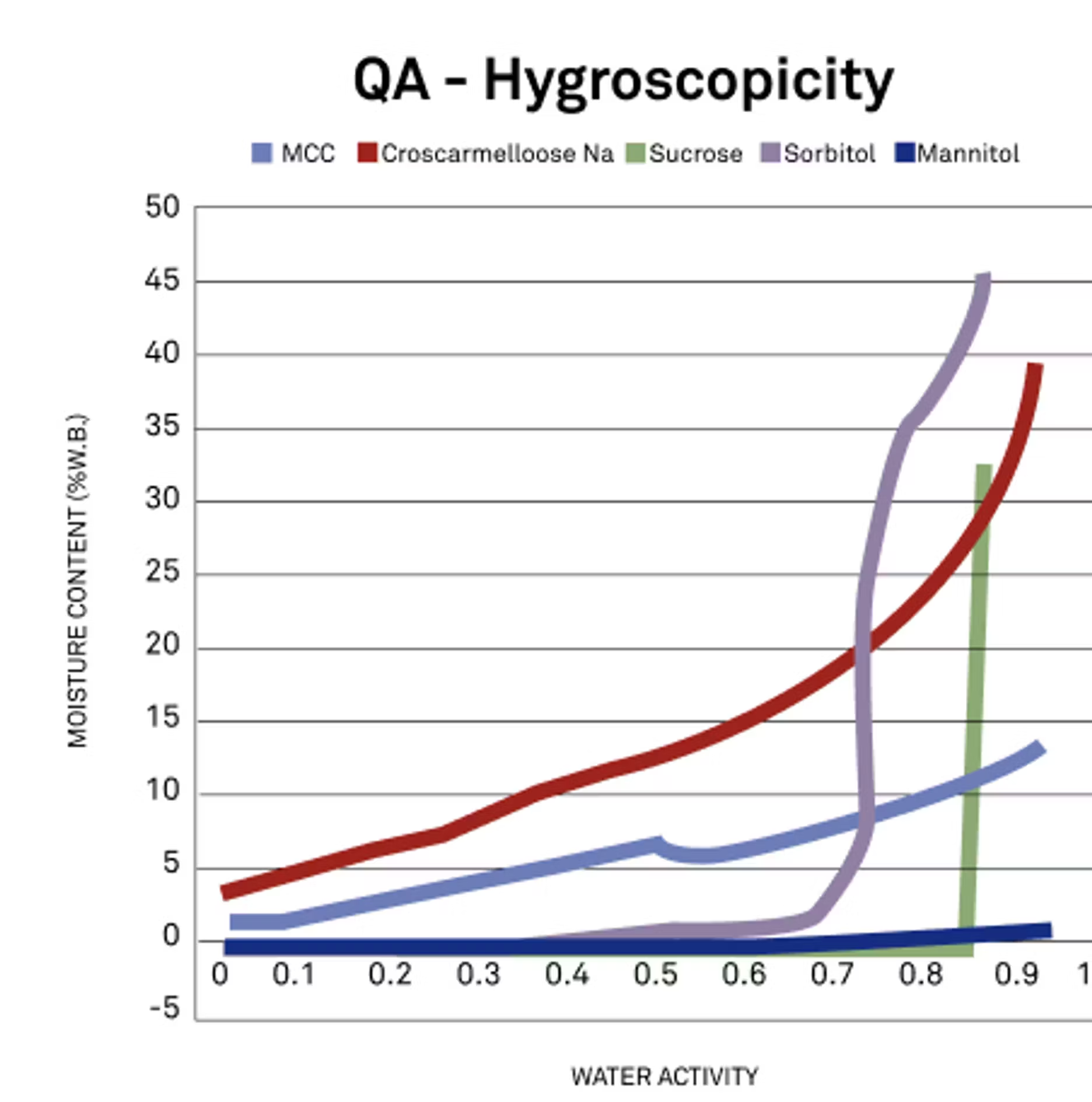
In this instance we're looking at different excipients, and the greater the slope of that curve, the higher the hygroscopicity and the more that the specific powder is picking up or absorbing water. Simply from looking at the shape of that curve, we can very quickly and visually see a difference in how hygroscopic those powders are. I don't know if there's anything you would add.
Mary:
That is a good explanation. Basically the steeper of that slope, the more hygroscopic it is, which also can mean it's going to be able to contribute more moisture to your final product, whatever that might be. That's definitely a factor is understanding that relationship and how hygroscopic something is.
Zachary:
I'll just also add that depending on your formula or depending on your goal or final outcome, you may want something that has a greater slope or not. It's just going to be product and formula specific, what type of slope you're looking for.
Mary:
If you want something that is going to increase or bind with the water as you wish, then that is a good way to do it. You can have an ingredient that has a steep slope will take on a lot of moisture and be able to add a lot of moisture to your product, and then you can formulate to the specific water activity you're looking at. But it's not necessarily a bad thing, it's just something to know.
2. What affected vitamin C degradation more, temperature or water activity?
Mary:
All right, I'll take this one. Water activity was definitely the major factor in the vitamin C degradation more so than the temperature. If you were to go back and look at the data, you could also see this for yourself because if you compare what those reactions with only varying the temperature versus when we varied the water activity, it's very clear actually for this one, which is the more important factor is water activity.
Zachary:
It's going to depend on each vitamin specifically, and something that we've actually been talking about recently. But even though vitamin C behaved in this specific way, you're going to have to look at each vitamin or each active ingredient, whatever you're concerned with it. This isn't always going to be the trend. It's not always going to be water activity having a bigger influence. It requires doing this type of study in order to understand which one will have the greater impact.
Mary:
Right. Because it could be oxygen or vitamin E that can go rancid. You could track that and you might have a different response for that. But for vitamin C it was water activity.
3. How does water activity or moisture content track with how quickly a powder will dissolve?
Mary:
I'm going to say it's not necessarily water activity or moisture content per se, it's more about the structure of the powder itself and how quickly it can dissolve and what's the environment that that powder is going to be in. If it's like a drink mix, then it is going to be in a high water activity atmosphere environment so that it'll dissolve quickly. But it depends. As an example, if you have something that has a hydrate, generally it's already got water bound to it that tends to dissolve quicker. It all depends. It's not as simple as knowing the water activity and moisture content of a powder, it's more about the structure and how that can quickly dissolve.
Zachary:
I'll add that by also looking at the isotherm and looking at a DVS test, if you're able to identify a deliquescence point or a point where everything goes into solution, then we can use a DVS test to understand how long is this going to take. Maybe looking at the structure and then also being informed by the isotherm as well.
Mary:
Right. That is an important part of a powder is having it dissolved because most of the powders we experienced are supposed to do that. If you just have a drink mix, you're going to add it to something else. But what if you want to prevent it to cake and clump before it's in the package? We've all experienced that or we've had a drink mix or something, and it's hard. Maybe we looked at the isothermal, we'll figure out what is that critical point for that mix and then make sure we have packaging that ensures it doesn't go above it, or maybe we need to add an anticaking agent or something like that. Those all factor into the solvability of a powder.
4. How does the shelf life of amorphous powder compare to the shelf life of crystalline powder?
Zachary:
It's going to depend on what is ending the shelf life. Are we looking at a texture change or a chemical change? First we'd have to define what does that end point look like, and then what would you add to that?
Mary:
Good question. With crystalline powders, they have a deliquescence point, which I mentioned. I know, again, going from a solid right to a liquid state, and that depends on the humidity they're exposed to. If you're able to keep the environment around that crystalline powder below that point, then it can change the activity rapidly and not change the structure of the powder. You can have really long shelf life. You can think of sucrose or NACL or something like that. You just have a long shelf life with that.
But if it's above it, then you've got trouble. It all matters unlike you said, what is going to end shelf life? What is the environment it's going to be exposed to? Then what makes sense? We did also a webinar on sweeteners, and so that was a interesting part about sweeteners and their characteristics of their dissolvability and we're switching between amorphous and crystalline structures and how that could precipitate out essentially out of a product at all is a complicated question. Is there a defined answer? Maybe I'll just try to simplify it. Crystalline structures, if you can keep it below that critical point, you can expose it to anything below that. Really it's not going to change because every water interaction is all like surface. But then, again, if it's above that or if it's a chemical reaction or if it's something else, then it's another thing we need to talk about.
Zachary:
I'll just add that for crystalline solids or powders, their deliquescence points are usually pretty high, maybe 0.9 water activity or somewhere in that range, whereas an amorphous powder may have a physical change 0.3 to 0.6 water activity. If it's on texture alone, if that is going to determine a shelf life, then I would imagine that most crystalline solids will have a longer shelf life. But if it's a chemical stability or chemical reaction that we're concerned with, it's going to take a deeper dive and an experiment to answer that question.
Mary:
Back with the other crystalline thing that we didn't mention as well is that if we have small particle sizes or we have odd structures or shapes or various sizes, that can impact where you'll start getting that bridging as a surprise at room humidity. Even saying that crystalline if you keep it below the deliquescence point that you're free, particle size would also definitely impact the stability of that crystalline structure.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
