Expertise Library
Shelf life simplified
Full-blown shelf life testing can be overwhelming, time consuming and expensive. Water activity measurements offer a far easier and more affordable way. Here's why.
Without an accurate, product-specific shelf life, you could be scrapping expired product that is still good. Or selling unexpired product that is actually bad. You could be paying too much for packaging that doesn’t help your product. Or giving up significant shelf life that would come from better packaging. The point is, you don’t know for sure because you’re operating in the dark.
So why don’t people do more shelf life testing?

Full-blown shelf life testing
Typically, it’s because a true, full-blown shelf life test is a daunting task. It involves complex relationships between moisture, temperature, and product failure modes.
Any number of things could make your product unsafe or unpalatable—mold, microbial growth, rancidity, changes in texture or flavor, vitamin degradation. Most people don’t have the expertise to do full-blown shelf life testing in-house, and getting an outside lab to do it is expensive.
There is a scientifically sound alternative to this kind of shelf life testing. It’s shelf life, simplified by water activity. It generates all the data you need to predict your product’s shelf life from an experiment anyone, even a small startup, can afford to run.
Shelf life and water activity
How does water activity simplify shelf life?
- It eliminates distractions. When you know your product’s water activity, you will know which failure modes are an issue for that product.
- It simplifies prediction. You can use your water activity meter plus one other measurement method (which depends on your particular failure mode) to run a straightforward, in-house experiment that will predict your shelf life accurately.
- It standardizes production. You can set a water activity specification that lets you achieve your optimal shelf life with every batch.
Your shelf life data can provide valuable insights to help you stop product failure, predict and lengthen shelf life, choose the most cost-effective packaging, and more.

How does water activity predict shelf life?
Water activity is an important means of predicting and controlling the shelf life of food products. Shelf life is the time during which a product will remain safe, maintain desired sensory, chemical, physical, and microbiological properties, and comply with nutritional labeling. Many factors influence shelf life, including water activity, pH, redox potential, oxygen, use of preservatives, and processing/storage conditions. By measuring and controlling the water activity of foods and pharmaceuticals, it is possible to:
- Predict which microorganisms will be potential sources of spoilage and infection
- Maintain the chemical stability of foods
- Minimize nonenzymatic browning reactions and spontaneous autocatalytic lipid oxidation reactions
- Control the activity of enzymes
- Prolong nutrients and vitamins in food
- Optimize the physical properties of foods
Factors that end shelf life
There are three main factors that influence shelf life: microbial properties, chemical changes, and physical deterioration. All of these factors are connected to water activity.
Microbial growth
Mold and microbial growth are the most dangerous threats to shelf life. Controlling water activity can inhibit or preclude microbial growth, extend shelf life, and allow some products to be safely stored without refrigeration. Using well-defined tables, you can set a water activity limit for your product and use this in shelf life testing.
Chemical degradation
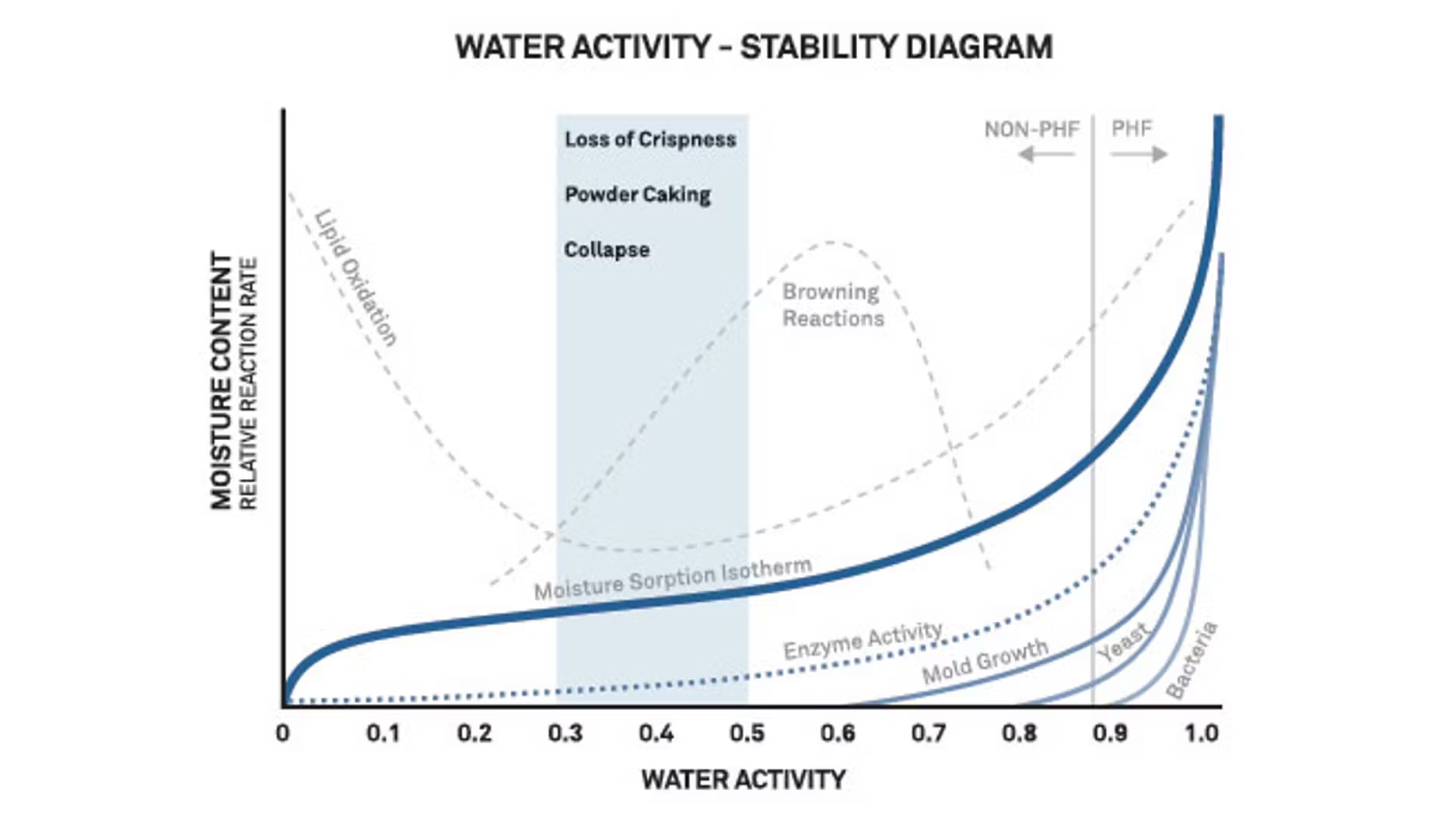
Water activity influences deteriorative chemical reaction rates because water acts as a solvent, can be a reactant itself, or can change the mobility of reactants through viscosity. For example, non-enzymic browning reactions increase with increasing water activity to a maximum at 0.6 to 0.7 aw, and lipid oxidation is minimized from about 0.2 to 0.3 aw. Optimum chemical stability is generally found near the monolayer moisture content, as determined from moisture sorption isotherms.
Physical deterioration
High and (less often) low humidity environments can affect a product’s water activity, causing undesirable changes in the product’s texture or physical properties and shortening shelf life. Issues include loss of crispness in dry products, caking and clumping of powders, and toughness or chewiness in moist products. Finding the critical water activity for your product can involve some research, but water activity makes it much easier to do.

Packaging, shipping, and storage
Water activity changes during shipping and storage can profoundly influence shelf life. Water activity is a function of temperature, and shipping and storage temperatures can affect water activity inside the package. Simplified shelf life testing can help you determine the best packaging and evaluate the effect of shipping and storage conditions on the shelf life of your product.
Get started with simplified testing
Information on how to maximize shelf life is in the food science literature, but it can be difficult to find step-by-step instructions. There are just a few things to keep in mind when developing a plan for testing the shelf life of your particular product.
DON’T try to analyze everything
There are many factors involved in shelf life, but the biggest impacts are water activity and temperature. Start by controlling those two factors.
DO determine the most likely failure modes
The shelf life of a product is typically impacted by just one or two failure modes. For example, the shelf life of potato chips is often ended by off flavors associated with lipid oxidation. Simplified shelf life testing should start by tracking lipid oxidation at various water activities and temperatures. Once the impacts of lipid oxidation have been considered, you can examine any other potentially limiting factors such as texture.
The basic steps to determining shelf life
- Control water activity and temperature
- Measure and track changes in the factors that end shelf life at chosen a and temperature
- w
- Collect data over time
- Identify the ideal water activity range for your product
Pick the right package
Once you have determined the ideal water activity range for your product, it’s time to consider packaging. The most significant determining factor for what will happen to your product’s water activity over time is the permeability of the packaging material—how well it can prevent moisture transfer under different conditions (see Wong et al 1999). To figure out correct packaging for a desired shelf life, you need two simple metrics: package permeability and critical water activity.
Find your WVTR
The driving force for water movement through packaging is a difference in water activity conditions inside and outside the packaging. Manufacturers use packaging to control the rate at which this water will move. Moisture is transferred through the packaging at a rate reported as water vapor transmission rate (WVTR). You can use the WVTR in mathematical models to determine optimal packaging for desired shelf life.
Identify your critical water activity range
One of the main goals of shelf life testing is to determine the best water activity range for your product. This may be a critical water activity which, if exceeded, will immediately cause safety or texture issues that end shelf life. Or it may be the “moisture sweet spot” which maximizes profit and eliminates potential taste, texture, and safety issues.
If physical changes are the primary mode of failure for your product, a dynamic dew point isotherm (DDI) curve may be able to identify the critical water activity. A DDI curve measures the change in sorption properties of a sample as it adsorbs and desorbs water (see Figure 1).

DDI curves can save a lot of time in identifying a critical water activity. You can obtain them by sending a sample of your product to METER Lab Services or by using the VAPOR SORPTION ANALYZER to develop your own DDI curves.
If microbial spoilage is the factor limiting your shelf life, you can identify a critical water activity or water activity range using limits that have been well established by research. Many microorganisms of concern are listed on this table showing the relationship between water activity and microbial growth.
If chemical factors such as lipid oxidation, Maillard browning, or vitamin loss are your product’s primary mode of failure, you will need to do a bit more work. Water activity is correlated to many of these chemical reactions, but you must experiment to determine what that correlation is for your particular product.
Package for shelf life success
Once package permeability and critical water activity are known, those values can be used to do predictive modeling.
Predictive modeling is often done through a series of complicated equations (described in the Additional Resources section), but there is a simpler way. A software program called the MOISTURE ANALYSIS TOOLKIT, will make these calculations for you. The toolkit will use these basic inputs to determine shelf life, set ideal packaging specifications, and even allow you to vary the analysis parameters to examine different packaging options.
Learn more about shelf life
In this 30 minute webinar, food scientists Mary Galloway and Zachary Cartwright talk about how to get answers to your shelf life questions. Learn how to:
- Troubleshoot issues and complaints to find out why shelf life is ending sooner than expected
- Predict how recipe changes will impact shelf life
- Compare the effect of different ingredient options
- Evaluate whether a specific packaging option will help you achieve or improve shelf life
Additional resources
ASTM International. ASTM E96-00 Standard Test Methods for Water Vapor Transmission of Materials. West Conshohocken, PA: ASTM International, 2000.
Azanha, A.B., and Faria J. A. F. “Use of Mathematical Models for Estimating the Shelf-life of Cornflakes in Flexible Packaging.” Packaging Technology and Science 18, no. 4 (2005): 171-178.
Carter, B.P., Galloway, M.T., Campbell, G.S., and Carter, A.H. 2015. The critical water activity from dynamic dewpoint isotherms as an indicator of premix powder stability. Journal of Food Measurement and Characterization. 9(4):479-486.
Carter, B.P., Galloway, M.T., Campbell, G.S., and Carter, A.H. 2015. The critical water activity from dynamic dewpoint isotherms as an indicator of crispness in low moisture cookies. Journal of Food Measurement and Characterization 9(3):463-470.
Carter, B. P., and Schmidt, S. J. “Developments in Glass Transition Determination in Foods using Moisture Sorption Isotherms.” Food Chemistry 132, no. 4 (2012): 1693-1698.
Risbo, J. “The Dynamics of Moisture Migration in Packaged Multi-Component Food Systems I: Shelf Life Predictions for a Cereal-Raisin System.” Journal of Food Engineering 58, no. 3 (2003): 239-246.
The Stability and Shelf-Life of Food, Edited by David Kilcast and Persis Subramaniam. Woodhead Publishing, 2000.
Koutsoumanis, Konstantinos, and George-John E. Nychas. “Application of a systematic experimental procedure to develop a microbial model for rapid fish shelf life predictions.” International Journal of Food Microbiology60, no. 2-3 (2000): 171-84. doi:10.1016/s0168-1605(00)00309-3.
Del Nobile, M. A., and Buonocore, G. G., and Limbo, S., and Fava, P. “Shelf Life Prediction of Cereal-based Dry Foods Packed in Moisture-sensitive Films.” Journal of Food Science 68, no. 4 (2003): 1292-1300.
Labuza, T.P., and Hyman, C. R. “Moisture Migration and Control in Multi-domain Foods.” Trends in Food Science & Technology 9, no. 2 (1998) 47-55.
Wong, Ee Hua, and Teo, Y. C., and Lim, T. B. “Moisture Diffusion and Vapor Pressure Modeling of IC Packaging.” Presentation at the Annual Electronic Components and Technology Conference, Seattle, WA, May 25-28, 1998.
Yuan, X., and Carter, B. P., and Schmidt, S. J. “Determining the Critical Relative Humidity at which the Glassy to Rubbery Transition Occurs in Polydextrose using an Automatic Water Vapor Sorption Instrument.” Journal of Food Science 76, no. 1 (2011) 78-89.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
