Expertise Library
Dynamic Vapor Sorption 101: What, why and how?

The water in food and pharma products impacts how they’re used, when they degrade, and more. Moisture can’t be safely ignored. Here's how dynamic vapor sorption (DVS) can help.
Dynamic Vapor Sorption 101: What, why and how?
Water is ubiquitous. Those who study the physical world or manufacture materials within it will eventually be forced to acknowledge moisture’s impact on the substances they work with.
More specifically, water – both in a material and around it – is a crucial factor in determining how and where a product or material can be used, when it will degrade, what (if any) treatments or coatings it needs, or if it needs to be entirely reformulated.
Moisture can’t be safely ignored – so how can its impact be measured and accounted for?
Through vapor sorption analysis.
What is dynamic vapor sorption (DVS)?
The goal of vapor sorption analysis is to learn how much of a solvent – usually water – is adsorped or desorped by a material and how quickly that happens.
To find this out, a sample of the material is placed in an environment where the amount of solvent vapor (humidity) can be controlled and adjusted. Changes in the sample’s weight are then measured and used to calculate how much vapor it adsorps or desorps.
Dynamic Vapor Sorption (DVS) is one popular way to analyze vapor sorption. Up until a few decades ago, a slow, manual process involving desiccators was the primary method of vapor sorption analysis. In 1991, Daryl Williams developed DVS to reduce the massive amount of time and manual labor that was required to get meaningful data.
How dynamic vapor sorption works
There are several types of DVS devices, but most are similar in their mechanism of action.
A typical Dynamic Vapor Sorption device holds a sample in a temperature-controlled chamber. It then uses humidified or desiccated air to bring the chamber to a set relative humidity level. Once the sample comes to equilibrium (assumed by weight) with the chamber’s relative humidity level, its change in mass is recorded. The device then repeats the process with increased or decreased relative humidity levels and records further changes. After the needed data points are collected, some DVS devices will use them to generate an isotherm.

Once a sample is prepared and inserted, dynamic vapor sorption devices perform vapor sorption analysis automatically. To record the same data before DVS technology required several desiccator enclosures, a tightly temperature-controlled room, and several weeks or months to rotate samples through different chambers and record the results
When and why dynamic vapor sorption is used
Competitive markets and tighter regulations have pushed companies in widely varied industries to research how their products respond to environmental conditions. This is likely why, in the 30+ years since its invention, Dynamic Vapor Sorption has been widely adopted.
Today, vapor sorption analysis continues to spread into new industries. Vapor sorption analysis most frequently answer questions similar to these:
- What conditions will cause a powdered material – whether a final product or a raw material – to cake, clump, and become unusable or unappealing?
- How well will any given packaging material protect a product from adverse shipping, weather or storage conditions?
- How long will a pharmaceutical, nutraceutical or supplement product’s active ingredient maintain effectiveness when exposed to fluctuations in humidity like those of a bathroom medicine cabinet?
Other, more niche applications include testing moisture’s effects on composite materials used in aviation, contact lenses and personal hygiene items, and many, many more.
DVS results and analysis, part 1: vapor sorption kinetics
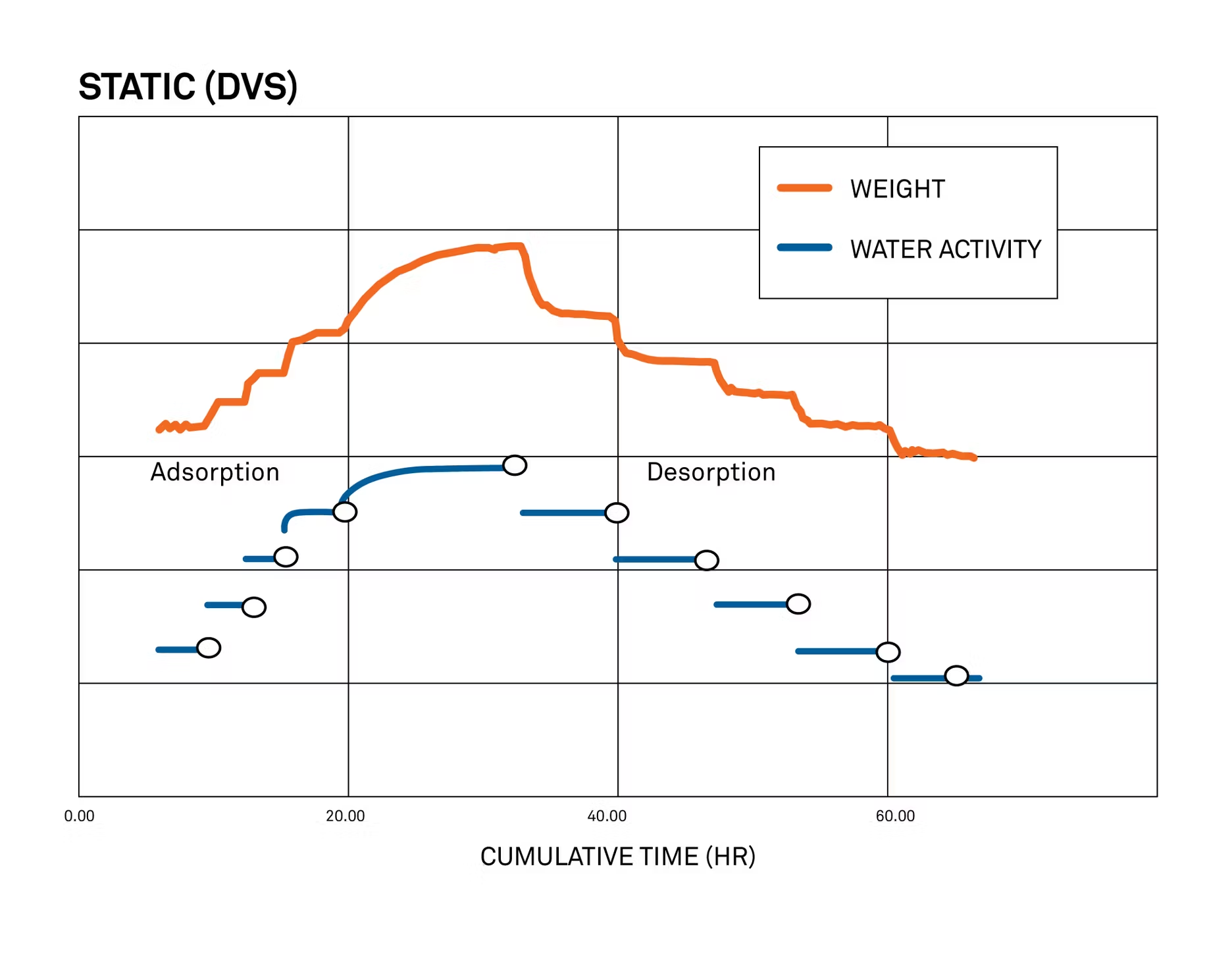
The results from a dynamic vapor sorption test are often visualized two different ways. The first, called vapor sorption kinetics, is all about timing. It graphs relative humidity levels in the chamber and changes in sample mass over time.
Stated another way, sorption kinetics show how quickly your sample will take in and release water from the surrounding environment. This is useful to know in cases where time is a critical factor, such as accelerated shelf life studies.
DVS results and analysis, part 2: vapor sorption isotherms
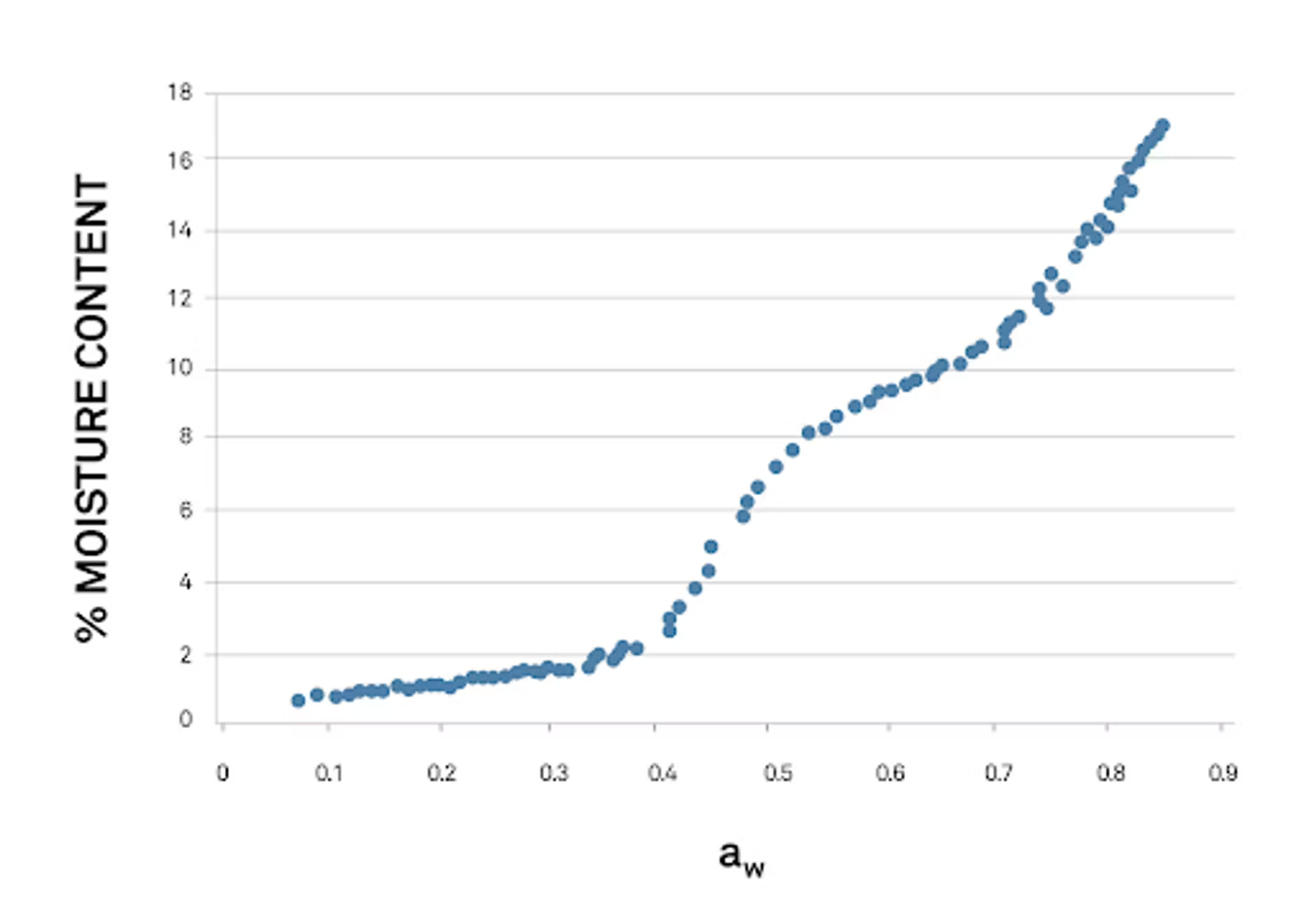
The other way to visualize vapor sorption data involves graphing the sample’s water activity (relative humidity) data on one axis and weight (sometimes moisture content is used instead) on the other.
These isotherms don’t emphasize how time influences the sample, but do show how mass or moisture content changes as in relation to relative humidity.
High resolution isotherms in this style make it simple to identify exactly where unwanted texture and quality transitions take place. These are often called “critical limits,” and show up as sudden jumps or drops in moisture content or mass. This information is crucial in many food manufacturing situations:
- In dry products like powders, it’s important to stay below an upper limit to prevent caking and clumping, but high enough that overdried, underweight product won’t corrode profit margins.
- In a cured meat snack, the critical limit window would ideally be low enough to prevent microbes, but high enough that the snack has a moist, palatable texture.
- In high-moisture food products like fruit bars, certain water activity levels are needed to prevent syneresis.
Adsorption, desorption and hysteresis explained
The difference between adsorption and desorption is important to note in both vapor sorption kinetics and isotherms.
Adsorption refers to a sample binding with moisture, pulling it out of humid surroundings. Desorption is when the sample releases moisture into dry or desiccated surroundings.
Few materials take up and release water in the same way or at the same rate. The difference in the adsorption and desorption is called hysteresis.
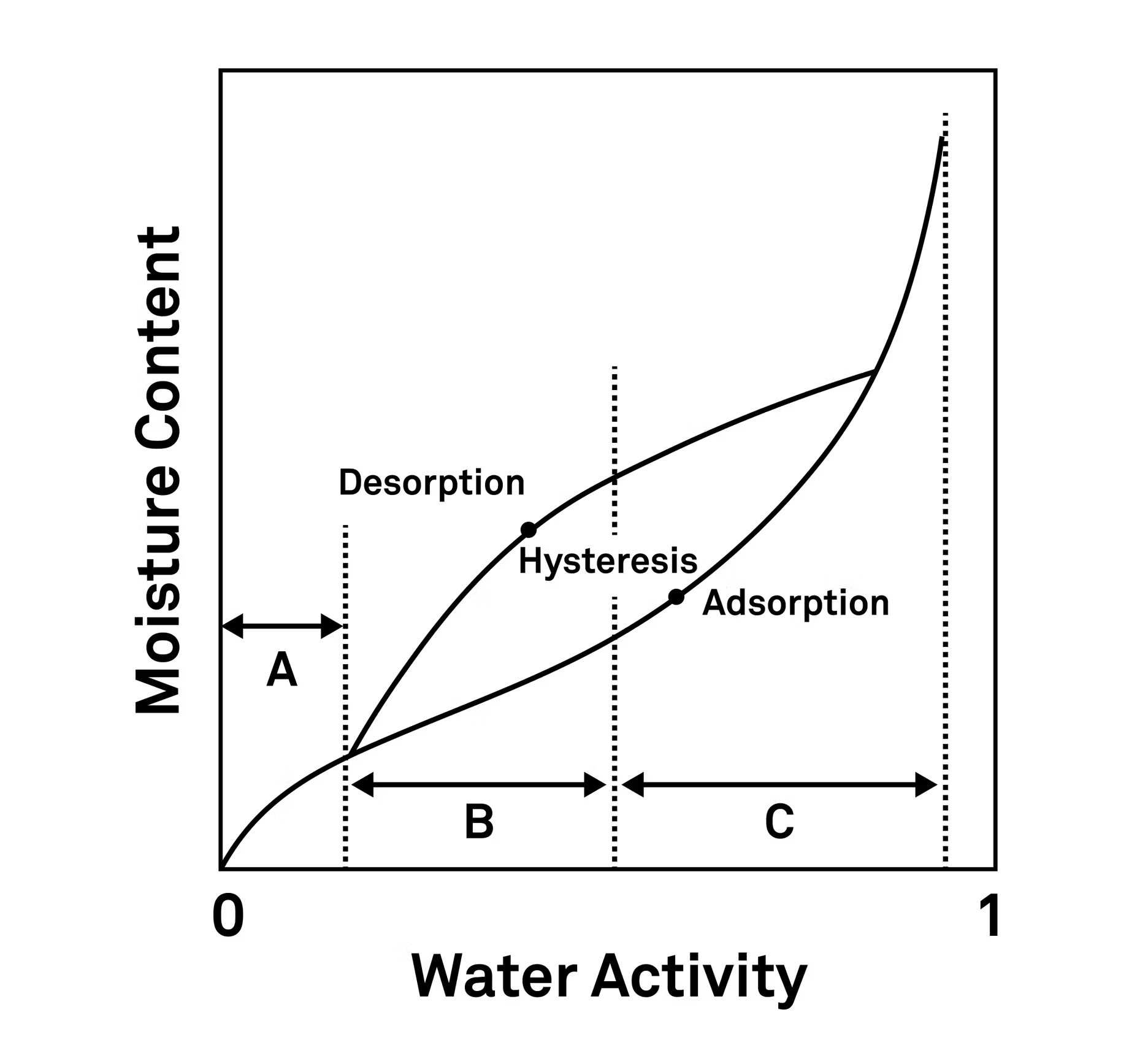
Hysteresis is an important concept to remember. Each adsorption/desorption cycle a material goes through changes the effects of future adsorption/desorption cycles. If a product has been taken past a transition point, its structure might have an irreversible change that can’t be reversed simply by drying it again.
Hysteresis is mostly used to understand a product, but it can also be used to evaluate the water holding capacity of a product as in the case of coatings, humectants, or new formulations.
DVS isotherm interpretation – a sample
Isotherm interpretation varies by application, but a breakdown of one method can help conceptualize others – in this case, an analysis of vapor-induced phase changes in spray-dried milk powder.
For more examples of how isotherms can be interpreted (to estimate shelf life, find the effectiveness of moisture-repellent films and coatings, and more) watch or read the transcript of our webinar, Understanding Isotherms.
The first step in interpreting this isotherm to study texture changes is to find the aforementioned critical water activity limits – the point where undesirable changes in texture begin to occur.
Using the second derivative of this sorption isotherm helps to highlight peaks on this curve. The peaks correlate to the water activity levels where the moisture content is increasing the fastest.
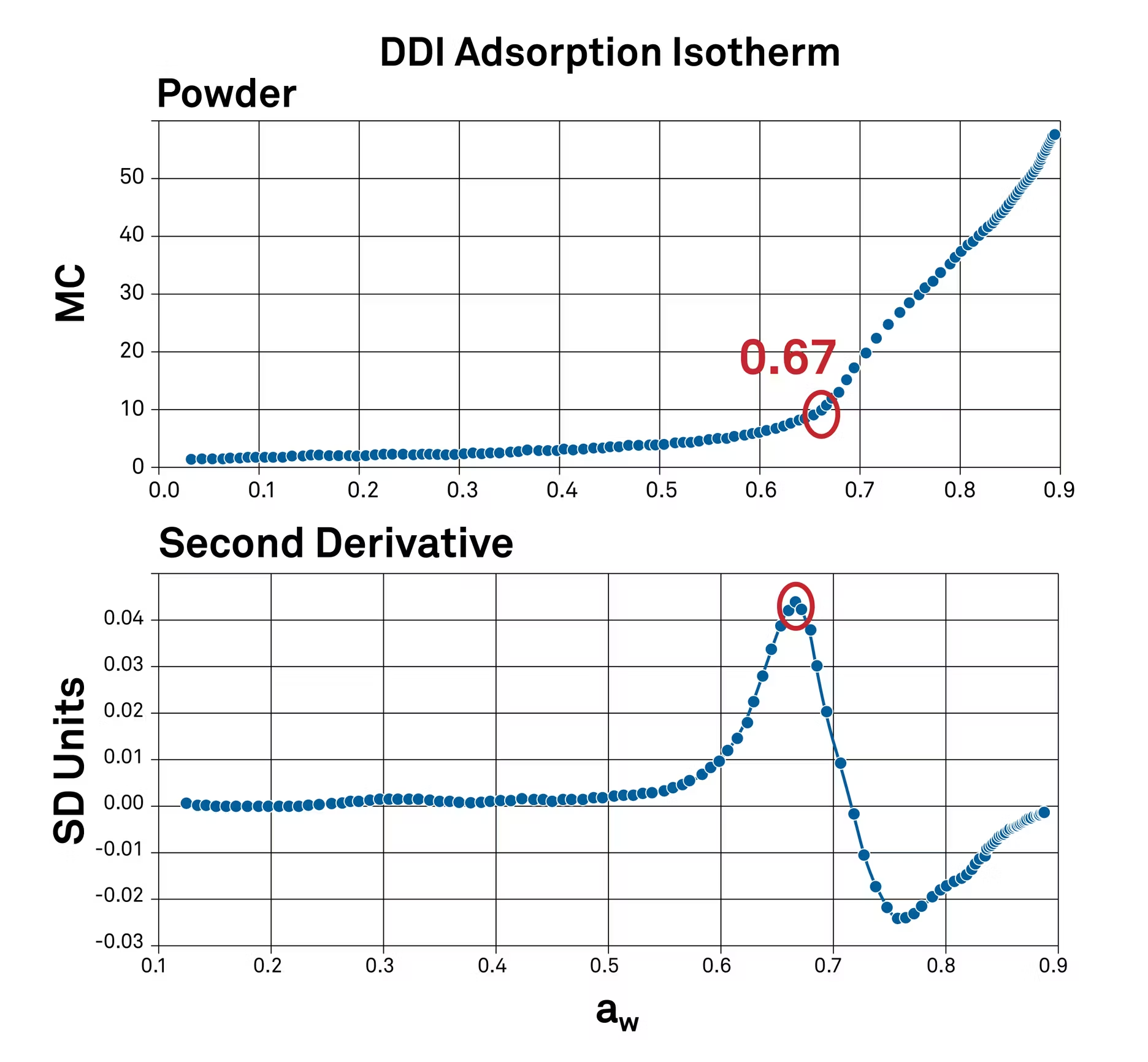
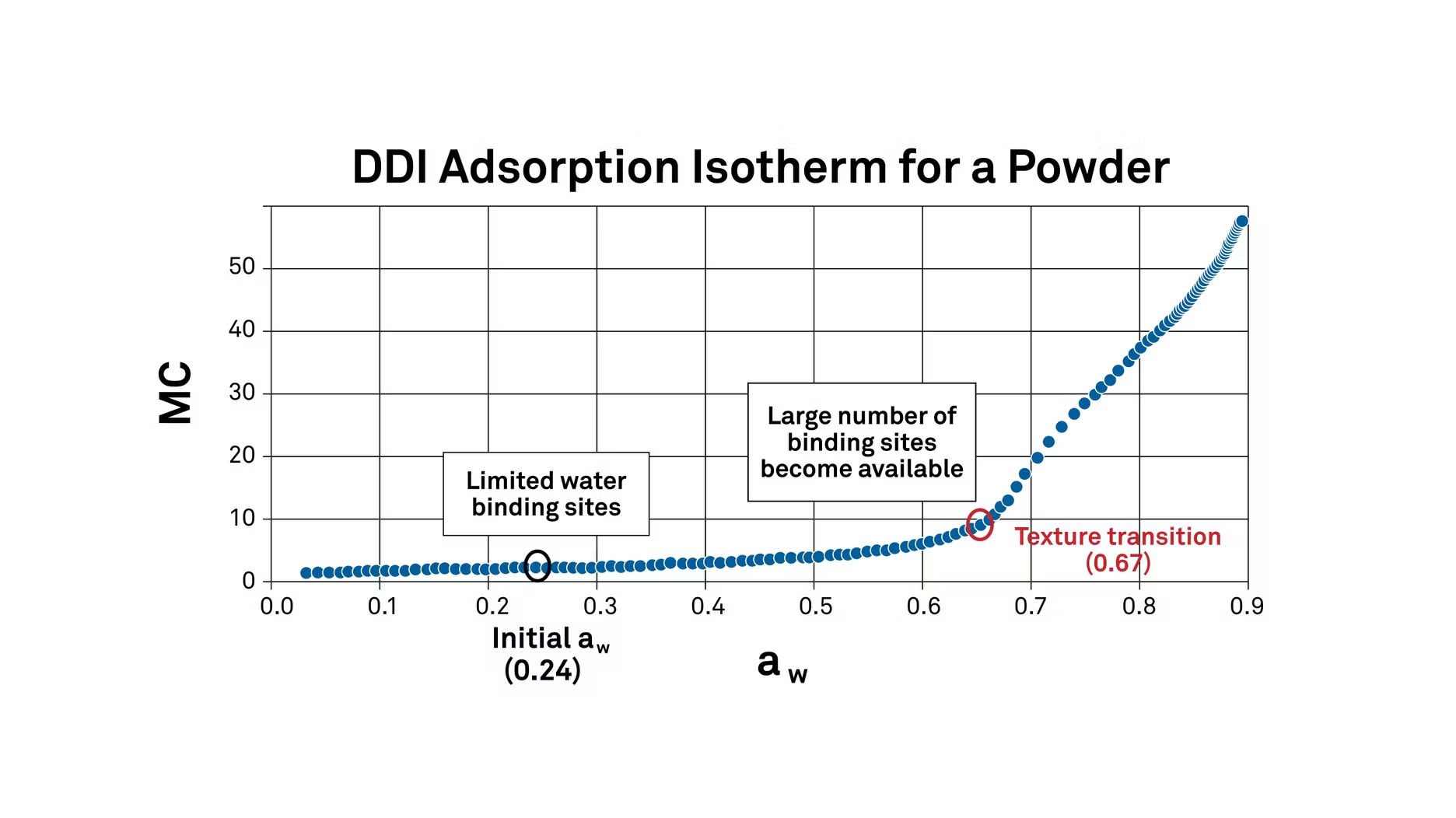
In this case, it's at 0.67 water activity. This means that 0.67 water activity or 67% relative humidity is a critical transition point for this powder, where its texture will change.
At low water activity levels, there are a limited number of water binding sites. But once it gets up to 0.67, the number of sites increases and more water can bind. As it moves even higher, severe caking and clumping begin (in this specific product). The isotherm shows exactly where these things will happen.
Water vapor sorption vs organic vapor sorption
The majority of Dynamic Vapor Sorption instruments are designed to study the sorption properties of water, some devices will also analyze how samples interact with organic vapors.
The goals and principles of the process remain the same: to learn how a sample adsorps and desorps vapor. In these devices, the sample chamber is filled with an organic vapor, not water vapor, at a predetermined humidity level.
DVS with organic vapors is most often used by material scientists developing chemical process control methods. It has also been found useful in helping the pharmaceutical industry develop stable and bioavailable active ingredients.
Dynamic vapor sorption instruments

A DVS device must be able to measure sample mass and expose the sample to humidified or desiccated air. After those consistencies, DVS devices vary widely in size, shape and capability. When shopping for a DVS device, consider the following:
- Device size. Some benchtop devices are very compact – approximately 30 cubic centimeters – while some multi-station devices can fill a large lab bench, or even be as large as a standalone cabinet.
- Read times. Some devices take days to create an isotherm. Some take weeks. Think through your sample throughput needs before purchase.
- Data resolution. Without high enough isotherm resolution, it is impossible to pinpoint critical transition points. Do you need low-resolution sorption kinetics or high-resolution isotherms?
- Sample size. Some DVS devices can precisely analyze samples as small as ten milligrams. Others require more sample for accurate analysis.
Other methods of vapor sorption analysis
Traditional vapor sorption analysis, performed by allowing samples to equilibrate in desiccators, is still performed in some labs and universities, despite the labor and equipment involved.

Those who choose this method will need several climate chambers, saturated salt solutions, and space to store them for quite a while. After preparing the materials and beginning the test, a lab technician will be required to remove samples from the chambers and weigh them regularly and record changes in mass until the desired data set is achieved.
This method takes consistent effort over time, and may not provide the detailed insight that other methods can.
Another alternative is the Dynamic Dewpoint Isotherm (DDI) method, a comparatively recent and little-known improvement upon the DVS method. Where DVS devices imitate the climate chamber method by using weight to judge when a sample has equilibrated to a specific humidity level, the DDI method equilibrates by the sample’s water activity.
DDI devices use humidified or desiccated air to change the relative humidity of the sample by a set interval (0.01 aw or 1% RH), allow the chamber to equilibrate to the new state of the sample, then record both the aw and weight of the sample. This process, repeated many times over a couple of days, results in isotherms with 100-150 or more data points than the DVS method’s 5-10, highlighting transition points and imitating fluid real-world conditions.
Newsletter signup
Case studies, webinars, and articles you'll love.
Receive the latest content on a regular basis!
